Cardiovascular imaging has become essential to achieving a better understanding of cardiovascular diseases. Due to the advent of new technology and the refinement of existing technologies, imaging's role has extended into the biological, functional, and hemodynamic diagnosis of multiple pathophysiologic processes. Current and future trends in cardiovascular imaging will focus on improving early diagnosis of vascular disease, so as to be able to promote cardiovascular health, and on its development as a useful tool in clinical decision-making. Imaging is also increasingly used to quantify the effect of novel therapies. The rapid development of molecular imaging and fusion imaging techniques improves our understanding of cardiovascular processes from the molecular and cellular points of view and makes it possible to design and test new preventive interventions. The proliferation and integration of imaging techniques in different clinical areas and their role in “translational imaging” plays an important part in the implementation of personalized therapeutic and preventive management strategies for patients with cardiovascular disease.
Keywords
.
INTRODUCTIONImaging has become the cornerstone in diagnosing cardiovascular disease (CVD). From its beginnings to the present day, there has been a revolution in the way we understand and study the heart. Until relatively recently, imaging was considered as no more than a means to visualize alterations in anatomy and structure. However, the discovery of new technology and improved understanding of classical techniques mean that it now plays a part in the biological, functional, and hemodynamic diagnosis of multiple pathophysiologic processes. Whole new areas in which noninvasive cardiovascular imaging can be used have opened up, ranging from early diagnosis to the study of the molecular and cellular mechanisms involved in multiple CVD and treatment assessment. This article focuses on areas in which we believe imaging will occupy a central role in the near future and on its potential contribution to optimizing the clinical and therapeutic management of individual cardiac patients.
IMAGING'S ROLE IN PRECLINICAL DIAGNOSIS AND THE PROMOTION OF CARDIOVASCULAR HEALTHThe prevalence of CVD is expected to increase in coming decades, leading to increased mortality and morbidity as well as considerable economic and social costs which will have to be borne by future generations. The present and future challenge for cardiology is to be able to detect subclinical CVD and thereby prevent some of its manifestations and reduce its impact on health. Imaging techniques can make a decisive contribution in this regard, particularly with respect to atherosclerosis and cardiomyopathies.
AtherosclerosisAtherosclerosis is a systemic disease with local manifestations. In its early stages, it is a silent disease; current technology can only detect it when it becomes more advanced. Algorithms based on traditional risk factors are useful for calculating cardiovascular risk but can be imprecise. For example, if we apply the usual criteria, approximately 30% of patients who suffer a major cardiovascular event risk would only have been classified as having an intermediate level of risk. Early detection of atherosclerosis may help to improve cardiovascular risk stratification. New imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), or applied molecular imaging not only make it possible to determine whether atherosclerosis is present but can also distinguish between stable plaque and plaque which is vulnerable or unstable. This can lead to improvements in risk stratification and therapeutic decision-making.
CT is useful in diagnosing and assessing the severity of atherosclerosis across all vascular territories and can also provide information on plaque composition (lipid, fibrous, or calcified) and eccentricity, and the presence of vascular remodeling.1,2 Its greatest contribution to the diagnosis of subclinical atherosclerosis is in the detection and quantification of coronary calcium using the Agatston score. This index has a high negative predictive value3,4 and adds independent prognostic value for cardiovascular risk above and beyond the classical risk factors.5 The amount of radiation required has decreased significantly with the new dose reduction protocols (<5 mSv for coronary angiography and <1 mSv for the study of coronary calcium). Likewise, iodinated contrast, which is contraindicated when there is significant renal impairment, is necessary in angiography but not for the study of coronary calcification.
When compared with histology, MRI provides very precise information on plaque size, presence of inflammation, and the condition of the fibrous layer.6,7 It can also indicate the presence of positive vascular remodeling. MRI has the advantage of not requiring radiation, which is important to its use in patient follow-up. Serial MRI studies have shown reduced atherosclerotic burden in the carotid region 12 months after initiating treatment with statinsc.8
PET can be used to quantify absolute coronary flow and coronary flow reserve, thereby providing information on coronary micro- and macrocirculation and, perhaps, on latent myocardial damage caused by cardiovascular risk factors.9 Finally, molecular imaging technology can be used to study the different stages of atherothrombosis pathophysiology at the cellular level. Because of this novelty and versatility, we decided to dedicate a section of this review to molecular imaging.
Several population studies have examined the usefulness of imaging techniques in the preclinical diagnosis of atherosclerosis. Some of these are finalized; others are ongoing. The ARIC (Atherosclerosis in Communities) study10 in the United States included 15800 patients and demonstrated an association between circulating levels of inflammatory markers, carotid wall thickness, and lipid core presence quantified using MRI. The MESA (Multi-Ethnic Study of Atherosclerosis) study included different imaging techniques such as cardiac MRI, carotid ultrasound, cardiac CT, and the ankle-brachial index, and led to many interesting results regarding the utility of cardiac imaging, particularly in regard to coronary calcium, and the progression of CVD.11,12 The primary objective of the ongoing U.S. BioImage study, which is part of the HRP (high-risk plaque) initiative, is to identify predictors of atherothrombotic events over a period of 6 years using imaging techniques. In Spain, 2 longitudinal general population studies are currently in progress to detect subclinical vascular lesions and monitor their progress using imaging techniques. The Santander Progression of Early Subclinical Atherosclerosis (PESA) study and the Aragon Workers Health Study13 are both led by the Centro Nacional de Investigaciones Cardiovasculares (CNIC, National Center for Cardiovascular Research). These studies evaluated more than 9000 apparently healthy individuals using different imaging techniques, including 2-dimensional and 3-dimensional vascular ultrasound of the carotid and femoral arteries and coronary calcium quantification with CT, at baseline, and at 3 and 6 years of follow-up. The main objective is to evaluate the onset/progression of silent atherosclerotic lesions. Specifically, the PESA-Santander (NTC01410318) study uses new hybrid imaging technology in addition to conventional techniques: subjects with evidence of silent atherosclerosis in imaging studies will be included in longitudinal studies using PET and MRI in CNIC's advanced facilities. This will allow both the presence/absence and volumetric progression of atherosclerotic lesions to be monitored, as well as their composition and inflammatory activity. These studies will provide important information on the role of imaging in detecting subclinical atherosclerosis and the impact this has on the prevention of cardiovascular events.
CardiomyopathiesImaging techniques are also useful for preclinical diagnosis of cardiomyopathy in at-risk populations, which may help in genetic counseling, formulation of lifestyle recommendations, or the decision to initiate or terminate certain therapeutic interventions. For example, the study of myocardial deformation using tissue Doppler and speckle tracking echocardiography can help detect early myocardial involvement in relatives of patients with familial hypertrophic cardiomyopathy. It can also contribute to the differential diagnosis for athlete's heart.14 These techniques have likewise proven useful in the early diagnosis and monitoring of cardiac toxicity from chemotherapy, thereby facilitating the suspension or modification of the therapeutic regimen during the period in which heart damage is reversible.15 In Chagas disease, the study of diastolic function and myocardial deformation using echocardiography16,17 and cardiac MRI18,19 can help detect early indeterminate or asymptomatic myocardial involvement in patients in whom antiparasitic treatment should then be prioritized. Other examples include the detection and monitoring of iron overload in the myocardium of patients with hemochromatosis, which has prognostic implications,20 and the ability to detect preclinical myocardial involvement in patients with Sarcoidosis. The latter can encourage early treatment with corticosteroids to stop the progression of the disease and improve prognosis.21
THE ROLE OF IMAGING IN TREATMENT DECISION-MAKINGThe last few decades have seen an increase in the range of medical, surgical, and percutaneous treatment options available to patients. Clearly, the most suitable therapeutic approach should be sought in each case to minimize complications and optimize outcomes. Moreover, improvements in surgical centers of excellence allow some interventions to be performed in asymptomatic or mildly symptomatic patients and in patients with more advanced heart disease who were previously considered inoperable. Clinicians may sometimes find it difficult to decide which patients will benefit most from a given intervention and when it is best implemented. In this respect, imaging techniques can provide crucial information. For example, in patients with anatomically intermediate coronary disease and stenosis, the new PET/CT scanners can integrate anatomical and functional information obtained after stress/rest myocardial perfusion. Delayed myocardial contrast-enhanced MRI sequences after administration of gadolinium or drugs (dobutamine or adenosine) provides essential information on viability and ischemia, information which can be used in decision-making on the appropriateness of coronary revascularization. Moreover, in patients with aortic or mitral insufficiency, increased contractile reserve with exercise or dobutamine before surgery predicts improved contractile function after valve surgery.22,23 In patients with severe aortic stenosis and severe ventricular dysfunction, assessment of contractile reserve by imaging provides vital information on myocardial recovery after surgery.24 Finally, MRI with delayed enhancement in patients with hypertrophic obstructive cardiomyopathy is associated with an increased risk of malignant ventricular arrhythmias,25 and can help clinicians decide whether an implantable cardioverter defibrillator is indicated in patients with one or two of the more relevant risk factors for sudden death. In patients presenting to an emergency department with chest pain and low or intermediate risk, quantification of coronary calcification and coronary CT angiography can be helpful in risk stratification and decision-making.26-28 Further studies are needed to establish whether studying plaque composition provides information that can be useful in deciding the most appropriate therapeutic strategy, as indicated by several earlier studies.29
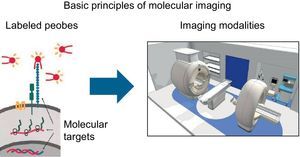
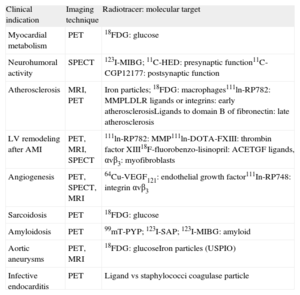
STUDYING THE PATHOPHYSIOLOGY OF BIOLOGICAL PROCESSES AT CELLULAR AND MOLECULAR LEVEL: MOLECULAR IMAGINGThe current trend is toward understanding the pathophysiologic mechanisms involved in CVD and identifying them early in order to develop new preventive therapies. This trend is likely to continue. Molecular imaging makes it possible to identify multiple markers in vivo, thereby allowing for noninvasive characterization and quantification of biological processes at the cellular and molecular level. This requires the availability of labeled probes (specific antibodies or ligands) that have a high affinity and specificity for similar structures (target molecules) and can detect them when using different imaging modalities (Fig. 1).30 The biochemical and radiophysical industries are focusing their attention on synthesizing and developing new radiotracers able to identify molecular, cellular, or specific genetic processes at high resolutions and whose use is safe for patients and easily accessible for cardiologists (Table 1).
Schematic image showing the process of molecular imaging performance. Adapted from Weissleder et al.30
Main Clinical Indications and the Imaging Modality and Radiotracer Used in Molecular Imaging
| Clinical indication | Imaging technique | Radiotracer: molecular target |
| Myocardial metabolism | PET | 18FDG: glucose |
| Neurohumoral activity | SPECT | 123I-MIBG; 11C-HED: presynaptic function11C-CGP12177: postsynaptic function |
| Atherosclerosis | MRI, PET | Iron particles; 18FDG: macrophages111ln-RP782: MMPLDLR ligands or integrins: early atherosclerosisLigands to domain B of fibronectin: late atherosclerosis |
| LV remodeling after AMI | PET, MRI, SPECT | 111ln-RP782: MMP111ln-DOTA-FXIII: thrombin factor XIII18F-fluorobenzo-lisinopril: ACETGF ligands, αvβ3: myofibroblasts |
| Angiogenesis | PET, SPECT, MRI | 64Cu-VEGF121: endothelial growth factor111In-RP748: integrin αvβ3 |
| Sarcoidosis | PET | 18FDG: glucose |
| Amyloidosis | PET | 99mT-PYP; 123I-SAP; 123I-MIBG: amyloid |
| Aortic aneurysms | PET, MRI | 18FDG: glucoseIron particles (USPIO) |
| Infective endocarditis | PET | Ligand vs staphylococci coagulase particle |
11C, carbon-11; 18FDG, 18F-fluoro-2-deoxy-D-glucose; ACE, angiotensin-converting enzyme; AMI, acute myocardial infarction; HED, hydroxyephedrine; LDLR, low-density lipoprotein receptor; LV, left ventricle; MIBG, meta-iodobenzylguanidine; MMP, metalloproteinase; MRI, magnetic resonance imaging; PET, positron emission tomography; PYP, pyrophosphate; SAP, serum amyloid P component; SPECT, single-photon emission computed tomography; TGF, tumor growth factor; USPIO, ultrasmall superparamagnetic particles of iron oxide; VEGF, vascular endothelial growth factor.
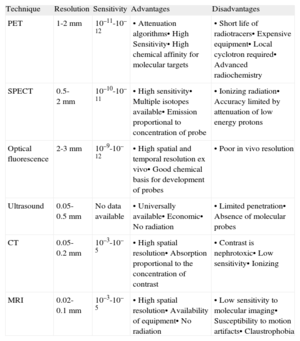
Molecular diagnosis originated from nuclear imaging techniques such as single-photon emission computed tomography (SPECT) or PET. This technology uses radioactive isotope molecules that generate an image from the photons emitted that reflects the distribution of the radiotracer in the body.31 Despite its high sensitivity for visualizing molecular structures, in recent years other new technologies have been developed without which the breakthrough in molecular imaging would not have been possible. These techniques include: MRI, which avoids radiation by using nonradioactive probes such as paramagnetic and superparamagnetic compounds and can also characterize tissue; CT, an ideal tool for the study of coronary lesions using iodinated compounds; ultrasound, which can detect microbubbles in the bloodstream using frequencies in the harmonic range; and fluorescence optics, which provide excellent spatial and temporal resolution ex vivo using fluorochromes. The selection of the ideal imaging technique depends on the biological properties of the system to be evaluated, the availability of molecular probes, and the technique's accessibility (Table 2). Integrated systems that combine imaging modalities with high spatial resolution (CT or MRI) and high sensitivity techniques such as SPECT or PET are also beginning to be used.
Advantages and Disadvantages of Different Imaging Modalities
| Technique | Resolution | Sensitivity | Advantages | Disadvantages |
| PET | 1-2 mm | 10–11-10–12 | • Attenuation algorithms• High Sensitivity• High chemical affinity for molecular targets | • Short life of radiotracers• Expensive equipment• Local cyclotron required• Advanced radiochemistry |
| SPECT | 0.5-2 mm | 10–10-10–11 | • High sensitivity• Multiple isotopes available• Emission proportional to concentration of probe | • Ionizing radiation• Accuracy limited by attenuation of low energy protons |
| Optical fluorescence | 2-3 mm | 10–9-10–12 | • High spatial and temporal resolution ex vivo• Good chemical basis for development of probes | • Poor in vivo resolution |
| Ultrasound | 0.05-0.5 mm | No data available | • Universally available• Economic• No radiation | • Limited penetration• Absence of molecular probes |
| CT | 0.05-0.2 mm | 10–3-10–5 | • High spatial resolution• Absorption proportional to the concentration of contrast | • Contrast is nephrotoxic• Low sensitivity• Ionizing |
| MRI | 0.02-0.1 mm | 10–3-10–5 | • High spatial resolution• Availability of equipment• No radiation | • Low sensitivity to molecular imaging• Susceptibility to motion artifacts• Claustrophobia |
CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography.
The main clinical applications for molecular imaging are likely to be in the assessment of metabolism and cardiac neurohumoral activity and the characterization of molecular processes critical to the development of many CVD, such as atherosclerosis, postinfarction ventricular remodeling, and angiogenetic processes associated with chronic ischemia.32 Molecular imaging could also play a role in the assessment of heterogeneous and difficult-to-diagnose conditions such as amyloidosis or cardiac sarcoidosis,33,34 as well as other more frequent conditions such as infective endocarditis or aortic aneurysms.35
Myocardial MetabolismPET and SPECT are the techniques most commonly used to study myocardial metabolism. Several useful radiotracers have been described, most notably natural substrates such as 11C (carbon-11) and similar substrates, such as 18F-fluoro-2-deoxy-D-glucose (18FDG). 11C has a short half-life (approximately 20 min), which means that a cyclotron and local chemical resources are required, while 18FDG has a long half-life (approximately 2 h), making it suitable for use in facilities lacking a chemical infrastructure. Because of its greater availability and the fact that it provides a higher-quality image by amplifying the cellular uptake signal metabolism, 18FDG has become the most widely used radiotracer for evaluating cardiac metabolic processes. For example, because it can detect the shift from an aerobic (fatty acids) to an anaerobic (glucose) metabolism, it is used to assess myocardial viability in the context of ischemic ventricular dysfunction. It is also used to characterize the inflammatory component of atherosclerotic plaque associated with increased glucose metabolism.36
Molecular Imaging of AtherosclerosisTo date, interest in molecular imaging has focused primarily on the study and identification of each phase of atherosclerosis. The aim is to facilitate early diagnosis, when the only changes are those in vessel morphology and composition, and preventive therapies can have some effect. The process of inflammation is critical in the formation, progression, and rupture of atherosclerotic plaque. The ability to visualize macrophages or other molecules involved in inflammation is making it possible to noninvasively detect early vulnerability or plaque instability. Early studies used superparamagnetic oxidized iron nanoparticles to detect macrophages with MRI,37 with possible applications in humans. It has also been shown that the concentration of macrophages in plaque is closely correlated with 18FDG uptake in PET imaging.38 Macrophages are a major producer of metalloproteinase (MMP) enzymes, which makes them a good choice as a molecular target. Recently 111-RP782 was used to study vascular remodeling after carotid injury in a murine model.39 It is also possible to visualize the low-density lipoprotein (LDL) receptor or adhesion molecules such as integrins and the VCAM-1 (vascular adhesion molecule). These molecules are critical in the early development of atherosclerosis and the proliferation and remodeling of vascular damage.40,41 These findings could be especially useful in the field of cell proliferation associated with vascular disease, such as in transplant vasculopathy or stenosis in the stent. It could also be possible to use new molecular probes such as fibronectin domain B ligands to identify angiogenesis and tissue repair, major markers of evolved atherosclerotic plaques.42
Molecular Imaging of Postinfarction Ventricular RemodelingThe postinfarct healing process starts early and continues with left ventricular remodeling, which is characterized by compensatory hypertrophy, dilatation of the ventricular chambers, and eventually ventricular dysfunction. Multiple regulatory processes are involved in ventricular remodeling, such as MMP, coagulation factor XIII and the renin-angiotensin system activation, along with the development and proliferation of myofibroblasts.43
Using noninvasive strategies, it is possible to visualize and quantify in vivo the activation of MMPs using radioligand MMP inhibitors such as 111-RP782.44 The identification of factor XIII could be useful in preventing ventricular dilatation and postinfarction heart rupture. Experimental studies have shown that the concentration of radioligand 111In-DOTA-FXIII increases in areas where there is increased activity of factor XIII, which leaves open the possibility of its use in humans.45 The renin-angiotensin system is activated when heart failure is present and is a possible target for molecular imaging. Initial studies have demonstrated the possibility of using angiotensin-converting enzyme inhibitors and AT1 inhibitors for this purpose (using 18F-fluorobenzoyl-lisinopril and 99mTc-AT1, respectively46). Currently, much experimental work aimed at using molecular imaging to prevent postinfarction ventricular remodeling centers on the myofibroblasts. These are considered ideal cellular components for visualization using ligands that target surface molecules such as tumor growth factor beta, αvβ3, or the angiotensin receptor.47
Molecular Imaging of AngiogenesisMyocardial ischemia results in cell hypoperfusion and hypoxia, which promotes the formation of new vascularization, or angiogenesis, from the proliferation of the pre-existing vascular bed. This process begins with several local and circulating factors, such as vascular endothelial growth factor (VEGF), and the interaction with extracellular matrix adhesion molecules, such as integrins. Using PET, SPECT, and MRI radiotracers that specifically target VEGF or integrins, it is possible to view the process of angiogenesis, which may have future implications in chronic heart ischemia and peripheral arterial disease. For example, good results have been obtained from the study of 111In, 64Cu -VEGF121, or monoclonal antibodies labeled against integrin αvβ3 in experimental models of myocardial and peripheral ischemia to identify and monitor angiogenesis.48-50
IMAGING'S ROLE IN ASSESSING NEW TREATMENTSToday it is possible to evaluate new therapeutic strategies in many diseases using cardiovascular imaging, notably in acute myocardial infarction, heart failure, and pulmonary hypertension.
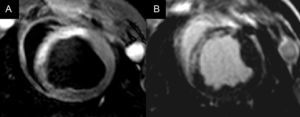
Early reperfusion and cardioprotective therapies aim to increase saved myocardium (defined as the proportion of non-necrotic area at risk) after acute myocardial infarction (Fig. 2).51 However, there are few methods to quantify the area at risk and which can be safely used in patients with acute myocardial infarction. In this sense, cardiac MRI is emerging as a promising technique and has been used in several clinical studies.52-54 However, expanding the use of MRI for this purpose is controversial.55 Questions remain about the best time to quantify the amount of saved myocardium or whether the new methodology can be used equally well for infarctions in different myocardial locations. Further studies will help to clarify these questions, which may have important implications for the design of clinical trials to evaluate cardioprotective therapies in acute myocardial infarction.
Magnetic resonance imaging scan (midventricular slice) in myocardial infarction, showing the area at risk on T2-STIR as a hyperintense region in the anterior septum (A) and the necrotic zone in late-enhancement sequences in the same location (B). The difference between the size of the area at risk and the myocardial necrotic area corresponds to the saved myocardium.
In the field of heart failure, of particular note is the potential importance of using specific MRI sequences to detect diffuse fibrosis, which could contribute to the clinical prognosis of different heart diseases.50,51 Future studies should evaluate whether quantification of diffuse fibrosis can be useful as an indirect objective in the study of new therapies.
In pulmonary hypertension, noninvasive imaging techniques may provide relevant information on 2 aspects, ie, the estimation and monitoring of blood pressure and pulmonary vascular resistance and the study and early detection of right ventricular involvement. This would be useful when monitoring patients on drug therapy because it would avoid the need for repeated cardiac catheterizations. With respect to the first point, Doppler echocardiography is now quite widely used to estimate pulmonary arterial systolic pressure from the peak velocity of tricuspid regurgitation flow, but it has limitations when there is a poor acoustic window or an absence of tricuspid regurgitation. Cardiac MRI is emerging as a promising technique in this context, as it can be used to assess and possibly monitor hemodynamic parameters.56,57 Future studies will clarify whether cardiac MRI use can be expanded to assess evolutionary hemodynamic changes in patients with the disease. Moreover, cardiac MRI is currently the gold standard in the study of the right ventricle and is postulated as a useful technique for the early diagnosis of right ventricular damage in patients with pulmonary hypertension.58-61
CARDIOVASCULAR IMAGING: NEW TECHNOLOGICAL ADVANCES AND TECHNICAL ASPECTS IN THE FUTUREThe extensive growth of bioengineering and technology is enabling a quantum leap in the development of new imaging equipment and the establishment of new clinical applications. These advances include the optimization of spatial and temporal resolution for each of the techniques, using lower amounts of radiation and improving safety.
In nuclear imaging, the commercial development of nanomolar teams such as micro-PET and micro-SPECT is noteworthy. With the micro-PET, it is possible to detect dynamic images with high sensitivity, use radioligands based on natural substrates, improve the algorithms for attenuation correction, and provide a precise quantification of molecular imaging. The micro-SPECT, on the other hand, allows the use of radiotracers with a longer half-life, permits the simultaneous display of multiple radiotracers, and improves on the spatial resolution of the micro-PET.62
Because of its limited resolution with respect to in vivo processes, optical imaging has been used primarily to evaluate ex vivo molecular processes. However, the development of 3-dimensional optical imaging or fluorescence molecular tomography and optical coherence tomography, which uses infrared light, has begun to resolve the problem of the limited penetration of light into tissue structures.63 There are even studies into the future role of optical imaging in the development of regenerative cell therapies and the detection of voltage and calcium in heart cells and tissues.64
In CT, technical development is oriented toward creating machines with multiple detectors that will reduce both acquisition time and the amount of ionizing radiation delivered, as well as optimizing tissue characterization by using dual-energy systems or spectral energy.65
In the field of cardiac MRI, 2 novel approaches stand out, both of which are promising. One of those is diffusion MRI, which provides 3-dimensional visualization of the myocardial and cardiac myofibrillar architecture through the diffusion of water molecules66 and could advance our understanding of cardiac contractility and remodeling processes, as well as postinfarction healing. The other technique currently being developed is MRI spectroscopy, which provides high-sensitivity information on cardiac metabolic processes using molecules such as C, Na, P, and Fl.67 The possibility of using a 3 T electromagnetic field could accelerate the use of spectroscopy to evaluate cardiac CVD.
However, this decade's biggest revolution in cardiovascular imaging and innovation is the development of hybrid technology or fusion, as evaluated in detail below.
Hybrid Technology: The Fusion of Anatomical-Functional Techniques and Their Clinical ApplicationsThe limitations of noninvasive imaging techniques that provide anatomical (mainly CT and MRI) and functional (SPECT and PET) information have led to the recent idea of combining several imaging modalities in one session. The possibility of simultaneously using several techniques can increase the low spatial resolution of nuclear techniques while increasing the sensitivity and reproducibility of the signal from molecular probes detected by anatomical techniques. This would help optimize the analysis and interpretation of images. Hybrid systems take advantage of each modality's strengths and can integrate molecular, anatomical, and physiological information in a single image.68
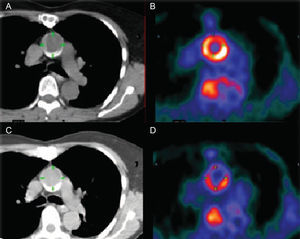
Currently available imaging modalities can be combined in different ways, but the most commonly used platforms are SPECT/CT, PET/CT, and PET/MRI (Fig. 3). The combination of nuclear imaging and CT is most often used in vascular studies because of the high spatial resolution provided (Fig. 4),69 while fusion with MRI is preferred in cardiac studies in which functional information is required (function, perfusion and/or late enhancement) and because it avoids the use of radiation associated with tomographic studies.70
Hybrid system combining positron emission tomography and magnetic resonance imaging. The 2 modalities share a rotating table that maintains the patient in the same position for positron emission tomography and magnetic resonance imaging and facilitates superimposition of images. MRI, magnetic resonance imaging; PET, positron emission tomography.
Images obtained with computed tomography (A and C) and positron emission tomography (B and D) of the ascending thoracic aorta before (A and B) and during anti-atherosclerotic treatment (C and D). Note the marked reduction in uptake of 18 fluorodeoxyglucose. Taken from Rudd et al.,69 with permission of the copyright owner.
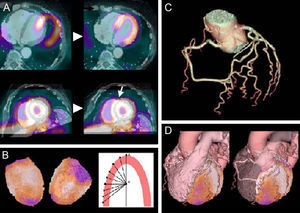
The added value of hybrid techniques stems from their ability to fuse structural and functional aspects. In this way, they make it easier to interpret the images and thus to locate the lesion and establish its physiological relevance (Fig. 5).70 It is essential that the 2 images overlap perfectly, as misalignment can lead to errors in determining the scope of diseased areas. Anatomical techniques must also be used to correct the soft tissue attenuation artifacts that affect nuclear imaging.
Postprocessing images from a positron emission tomography/computed tomography hybrid system. A, simultaneous acquisition of both positron emission tomography and both computed tomography images; B, detection of epicardial contour; C, segmentation of the coronary arteries; D, 3-dimensional reconstruction. Taken from Kaufman et al.,70 with permission of the copyright owner.
The main clinical contributions of this new technology include the possibility of establishing the pathophysiologic significance of anatomically moderate atherosclerosis by myocardial perfusion.71 For example, Rispler et al.72 demonstrated better specificity (63%-95%) and similar sensitivity for the detection of coronary artery disease with SPECT/CT compared to conventional angiography. Clinical studies are being developed to assess the diagnostic and prognostic value of PET/MRI for atherosclerotic disease in multiple vascular territories (carotid, aorta, iliac, and femoral). Having an MRI study simultaneously available with the PET image means that the intrinsic components of the plaque can also be characterized with T1 and T2 sequences. It is not yet clear what type of patient will benefit from this new technology, but it will likely be those with intermediate cardiovascular risk or those in whom it is not clear whether revascularization is indicated or not.
Technology can also play a role in studying the inflammatory changes involved in acute myocardial infarction. This is particularly true for PET/MRI, which makes it possible to simultaneously evaluate cardiac anatomy and function, the extent of necrosis, and myocardial perfusion and metabolism. Preclinical studies are currently in progress to compare the behavior of remote myocardium with infarcted myocardium.73 Hybrid techniques can also be used to explore, guide, and monitor local cellular and genetic therapies for several conditions.74
In conclusion, all of these new integrative systems are capable of noninvasively and simultaneously assessing anatomical and biological features, and offer enormous potential to advance molecular imaging. Over time, it will be necessary to define candidate patients, establish protocols for acquisition and interpretation, reduce radiation doses in equipment using ionizing radiation, evaluate the overall cost-effectiveness of the techniques, and validate them for a wide range of applications in large clinical trials before implementing the new technology in clinical practice.
PROLIFERATION AND INTEGRATION OF IMAGING TECHNIQUES IN DIFFERENT HEART UNITSIn recent years, we have seen 2 major changes that will become even more evident in the future. On the one hand, echocardiography units have become multimodal imaging units which include not only all forms of echocardiography (Doppler echocardiography at rest, exercise and pharmacologic stress echocardiography, 3-dimensional and transesophageal echocardiography), but also CT, cardiac MRI, and nuclear imaging. This change has important implications. First, imaging specialists must be trained in several of these techniques, which requires extensive postresidency training. Second, the attending physician must be aware of the characteristics of each technique, including cost, safety profile, indications, contraindications, potential, and limitations, so as to be able to decide, together with the cardiac imaging specialist, which technique is most appropriate in each case.
The second change is that imaging techniques have left the echocardiography laboratories and radiology units and become part of all specialized cardiac units. Complex electrophysiology procedures such as ablation of the pulmonary veins are today performed using cardiac mapping after cardiac CT or MRI. Complex cardiac hemodynamic studies such as the implantation of percutaneous or transapical prostheses, or the closure of atrial septal defects or periprosthetic leaks, require support from transesophageal echocardiography. Similarly, in cardiac surgery operating rooms valve repair is inconceivable today without transesophageal echocardiography. This means that different specialists, not just imaging specialists, need to be familiar with the imaging techniques they rely on. In the near future, training in each subspecialty, as well as in cardiac and vascular surgery, will include specific training in cardiovascular imaging.
TRANSLATIONAL IMAGING: THE NEXUS BETWEEN ANIMAL AND CLINICAL EXPERIMENTSCardiovascular imaging plays a key role as a link between animal experiments and clinical studies because it makes it possible to practice effective translational medicine. The ability to visualize pathophysiologic processes in vivo using imaging techniques in animal models helps us to understand the basis of a disease and allows for improvements in patient diagnosis and treatment. This close relationship between experimental and clinical settings can be 2-way, whereby what we learn from animal models can be applied to patients but we can then go back to animal models to understand the mechanisms involved. It is essential to define both the key questions and the methodology that will lead to the correct answers, and these are areas where imaging plays a vital part.
Some advantages of using imaging techniques in animal experiments include the widespread availability of established models of CVD, the possibility of carrying out serial studies and postmortem evaluation, and their low cost when compared to clinical studies. They can also be used in the validation of new therapies, as well as to facilitate the design and development of radiotracers and innovative imaging equipment, and to expand our knowledge of the mechanisms of action of certain chemicals. It should be noted that many experimental studies have led to the design of clinical trials which use the same imaging technology, which thereby serves as a translational link.75 For example, imaging techniques made it possible to reassess the role of beta blockers in myocardial infarction; previous experimental studies76,77 using cardiac MRI demonstrated that metoprolol increases the amount of saved myocardium and reduces reperfusion damage. These findings have led to the organization of a clinical trial (METOCARD-CNIC: NTC01311700),78 whose main objective is to show that early administration of beta blockers, prior to reperfusion, reduces infarct size in patients with acute myocardial infarction. The study uses MRI methodology to quantify the biological effect of therapy in the same way as in preclinical studies.
Imagining the Future of Cardiovascular ImagingThe unstoppable development of new imaging technology is bringing about a change in the way we confront CVD. In the same way that collecting blood samples and analyzing the concentration of different substances became the cornerstone of diagnosis for decades, noninvasive imaging will lead to similarly major change. Being able to evaluate anatomical and biological processes noninvasively represents a paradigm shift that we will only be able to properly appreciate with the perspective of time.
FUNDINGB. Ibáñez has several sources of competitive funding which are relevant to the subject of this review: CNIC-traslational grant (01-2009); Fondo de Investigación Sanitaria (PI10/02268); Fomento de Investigación Clínica Independiente (EC10-042), and Fundación Mutua Madrileña (AP8695-2011).
CONFLICTS OF INTERESTNone declared.