Technological advances over the past decades have allowed improved diagnosis and monitoring of patients with acute coronary syndromes as well as patients with advanced heart failure. High-quality digital recordings transmitted wirelessly by cellular telephone networks have augmented the prehospital use of transportable electrocardiogram machines as well as implantable devices for arrhythmia monitoring and therapy. The impact of prehospital electrocardiogram recording and interpretation in patients suspected of acute myocardial infarction should not be underestimated. It enables a more widespread access to rapid reperfusion therapy, thereby reducing treatment delay, morbidity and mortality. Further, continuous electrocardiogram monitoring has improved arrhythmia diagnosis and dynamic ST-segment changes have been shown to provide important prognostic information in patients with acute ST-elevation myocardial infarction. Likewise, remote recording or monitoring of arrhythmias and vital signs seem to improve outcome and reduce the necessity of re-admissions or outpatient contacts in patients with heart failure or arrhythmias. In the future telemonitoring and diagnosis is expected to further impact the way we practice cardiology and provide better care for the patient with cardiovascular disease.
Keywords
.
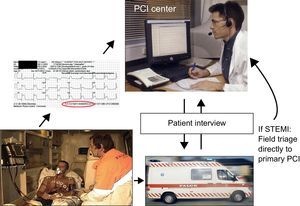
INTRODUCTIONPatients with ST-elevation myocardial infarction (STEMI) should receive reperfusion therapy as soon as possible to reduce mortality and morbidity. Guidelines recommend primary percutaneous coronary intervention (PCI) as the preferable strategy, if this can be performed in a timely manner.1,2 One of the main obstacles to a more widespread use of primary PCI is the limited accessibility to PCI centers operating on a 24-h basis. Thus, to treat STEMI patients within the recommended time limits, early diagnosis is essential in order for patients to be transported to a PCI center in due time. This has led to an increased interest in prehospital diagnosis. The diagnostic modality of choice has been the electrocardiogram (ECG). The ECG is recorded in the field and interpreted by emergency physicians, paramedics or cardiologists receiving the ECG in the hospital by wireless transmission (Figure). This method is very robust and has been successfully implemented in several countries worldwide.3–9 Prehospital ECG diagnosis and field triage directly to primary PCI has been shown to decrease treatment delay significantly and is associated with a lower mortality.10,11
An example of the set-up for prehospital electrocardiogram diagnosis. The electrocardiogram is recorded in the ambulance and sent wirelessly to a primary percutaneous coronary intervention center. The electrocardiogram is interpreted by the on-call cardiologist and after talking to the patient and/or paramedic a decision is made to either redirect the patient for primary percutaneous coronary intervention, in case of ST-elevation myocardial infarction, or send the patient to the nearest local hospital for further diagnosis and initial pharmacological therapy. PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
The prehospital use of continuous ECG surveillance in the form of ST monitoring has been shown to provide important prognostic information and might further improve triage for tailored treatment regimens.12 Both ST monitoring and ECG diagnosis rely on optimal equipment for ECG recording. Through the years new hardware and analysis algorithms along with more practical lead positioning systems have been introduced and might help to further expand the indications and use of prehospital ECG recording and diagnosis.
PREHOSPITAL ELECTROCARDIOGRAM RECORDINGIn the 1970s Uhley reported their experiences in transmitting single-lead telemetry from the ambulance to the hospital for arrhythmia monitoring in patients with suspected cardiovascular disease.13 For several years, however, it was not possible to transmit a diagnostic-quality 12-lead ECG due to technical difficulties and unstable data networks. In 1987 Grim et al. published a paper on successful implementation of diagnostic-quality, 12-lead ECG transmission using new error-correcting digital transmission via cellular phone links.14 The new transmission technology was validated in other studies, showing the possible benefit of early and reliable diagnosis of acute myocardial infarction.15,16
In the late 1990s the terms STEMI and non-ST-elevation acute myocardial infarction were introduced to ensure a more clinically and therapeutically relevant triage of acute myocardial infarction patients. This primarily relied on the large thrombolysis trials showing an improved outcome with the immediate use of lytic therapy for patients with STEMI.17,18 Early reperfusion therapy thereby became an important objective in the optimal treatment of STEMI and this led to a further interest in early diagnosis and optimal triage. In 2000 Wall et al. reported that prehospital diagnosis of acute myocardial infarction was associated with more rapid reperfusion therapy by thrombolysis,19 but the focus was still mainly on the in-hospital, emergency department delays in diagnosis.
However, this changed as several randomized trials and meta-analyses proved primary PCI to be a superior reperfusion strategy in STEMI patients, if performed in a timely manner.20–23 The limited access to PCI centers increased the focus on early diagnosis, allowing the patient direct admission or transfer to a PCI center as soon as possible.
In 2005 Terkelsen et al. demonstrated the benefit of prehospital ECG diagnosis and field triage to a primary PCI center in patients with STEMI. The study comprised 161 patients. A prehospital ECG was recorded in 106 patients, 21 of them admitted directly to a primary PCI center. The time from first medical contact to balloon was a median 81min shorter in patients with prehospital diagnosis and field triage compared to patients diagnosed in the hospital.24 Prehospital ECG diagnosis and field triage not only allows the patient to bypass the emergency room of a local, non-PCI-capable hospital, it also reduces in-hospital delays, as the catheterization laboratory is able to prepare for the imminent arrival of the patient. Sejersten et al. have further demonstrated this.6 In their study, door-to-PCI delay was significantly reduced in patients with a prehospital diagnosis compared with inhospital diagnosis (34 vs 97min, P<.001).
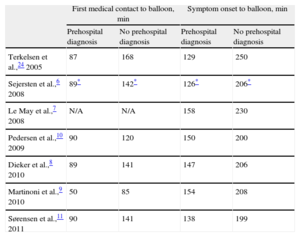
Studies from many countries have reproduced these results despite varying geographic settings, health-care organizations and prehospital logistic set-ups (Table 1).
Time From First Medical Contact or Symptom Onset to Balloon in Recent International Publications on Prehospital Electrocardiogram Diagnosis and Triage.
| First medical contact to balloon, min | Symptom onset to balloon, min | |||
| Prehospital diagnosis | No prehospital diagnosis | Prehospital diagnosis | No prehospital diagnosis | |
| Terkelsen et al.,24 2005 | 87 | 168 | 129 | 250 |
| Sejersten et al.,6 2008 | 89* | 142* | 126* | 206* |
| Le May et al.,7 2008 | N/A | N/A | 158 | 230 |
| Pedersen et al.,10 2009 | 90 | 120 | 150 | 200 |
| Dieker et al.,8 2010 | 89 | 141 | 147 | 206 |
| Martinoni et al.,9 2010 | 50 | 85 | 154 | 208 |
| Sørensen et al.,11 2011 | 90 | 141 | 138 | 199 |
N/A, not available.
Recently Sørensen et al. evaluated the routine use of prehospital ECG diagnosis and field triage in a larger region comprising both urban and rural surroundings.11 The authors found that prehospital diagnosis and triage reduces the importance of distance to primary PCI center, in as much as time from first medical contact to balloon inflation only varied 9min between patients belonging to urban versus rural hospitals, with a median 10km versus 40km distance to the PCI center (Table 2). Further the study showed a decreased mortality in patients with prehospital diagnosis and triage. A recent Dutch study further emphasized the importance and value of prehospital diagnosis for patients living far from a PCI center.25
ELECTROCARDIOGRAM BODY-SURFACE MAPPINGOne of the limitations of the 12-lead ECG, whether used in-hospital or in the prehospital phase, is the lack of sensitivity. Not all anatomic parts of the left ventricle are equally well covered by conventional leads. The infarct location most commonly missed on 12-lead ECG is the inferoposterior myocardial infarction. It was established many years ago that this limitation can be overcome by using additional leads, like V7-V9.26 Actual body-surface mapping often uses an 80-lead ECG, which improves the ability to detect acute myocardial infarction and thus improve the sensitivity of electrocardiography in large clinical series.27 The sensitivity for detection of troponin-confirmed acute myocardial infarction approaches 80% and c-statistics improve with a factor of almost 0.10.27 Right ventricular involvement complicating inferior STEMI can also be better detected by 80-lead body-surface mapping ECG.28 Recently a randomized trial compared the prevalence, clinical care patterns, and clinical outcomes of patients with STEMI identified on 80-lead but not on 12-lead (80-lead-only STEMI). The primary outcome of the trial was door-to-sheath time in patients with 80-lead-only STEMI versus patients with STEMI identified by 12-lead alone (12-lead STEMI). The 80-lead ECG provided an incremental 27.5% increase in STEMI detection versus the 12-lead. Patients with 80-lead-only STEMI have adverse outcomes similar to those of 12-lead STEMI patients but are treated with delayed or conservative invasive strategies.29
The 80-lead ECG body-surface map has also been used in the prehospital phase despite being more time-consuming to mount. Even in this setting the sensitivity of the acute myocardial infarction detection method is comparable to findings in the more protected in-hospital setting.30 Despite these findings, body-surface mapping for evaluation of patients with chest pain has not gained widespread usage. This could change with the invention of easy to apply technologies, which are appearing for commercial use.
NOVEL ELECTROCARDIOGRAM RECORDING AND LEAD SYSTEMSAs the prehospital setting often implies working under difficult circumstances with patients in a very poor clinical status, there has been some focus on developing ECG monitoring systems for simple, fast application with a durable design.
One such system (the EASI lead system), using only 4 leads to extract a full, diagnostic “12-lead” ECG, has been compared with a conventional 12-lead ECG by Sejersten et al.31 The authors report that the 4-lead EASI system was just as good for detecting acute, myocardial ischemia as the standard 12-lead ECG. Drew et al. also investigated the use of a more simple lead system in the Synthesized Twelve-lead ST Monitoring and Real-time Tele-electrocardiography initiative. The 5-lead ECG system used in this series of studies has proven effective in diagnosing ischemia and has reduced treatment delay in the study region (Northern California, United States).32,33
TELEHEALTHCARE FOR PATIENT AND DEVICE MONITORINGThe combination of an aging population and continued developments of modern therapy within cardiology and internal medicine in general have led to an increasing prevalence of chronic diseases, including heart failure,34 and the need for implantable devices.35 This development has increased the need for continued and close contact between the patient and the healthcare system to ensure the optimal treatment for the individual. Consequently, the healthcare system is challenged by the still-increasing need for qualified healthcare professionals, and resources in general, as well as increasing costs. One way to approach this challenge is the use of telehealthcare, allowing the patient to be in close contact with the appropriate healthcare provider and exchanging valuable information needed for managing the patient's disease.
Telehealthcare is defined as personal healthcare over a distance and consists of the following components: a) data provided by the patient (eg, blood pressure, pulse, weight, subjective information about health); b) electronic transmission of data from the patient to a healthcare professional, and c) personalized feedback tailored to the individual patient.36 Telehealthcare can be based on either real-time (synchronous), store-and-forward (asynchronous), or hybrid systems.37 Synchronous telehealthcare requires the availability of patient and healthcare provider at the same time and real-time processing of the patient's data, while asynchronous telehealthcare provides more flexibility for both the patient and healthcare provider.
The technology used in telehealthcare is a composite of portable medical imaging devices (eg, blood pressure device), computer/smart phone, and the wireless communication infrastructure, requiring a stable network for data transmission.38 The transmitted data may then be stored at a receiving station for momentary or later processing and review.
Four generations of telehealthcare for remote management of patients have been proposed38: a) 1st generation), a system of nonreactive data collection and analysis, with asynchronous transmission of data to a healthcare provider, eg, from an event recorder; b) 2nd generation, a system with a non-immediate analytical or decision-making structure, with synchronous data transfer, allowing the healthcare provider to view data and recognize important changes, but delays may occur if the system is only active during the day; c) 3rd generation, a remote patient management system with consistent analytical and decision-making support by a healthcare personal, even outside of office hours, and d) 4th generation, a fully integrated remote management system, where data from both invasive and noninvasive telemedical devices are linked in a telemedical platform and available for both the primary care provider and the telemedical center.
A wide variety of both noninvasive and invasive variables are available for telehealthcare within cardiology. Most are recorded manually by the patients via a device (eg, systolic and diastolic blood pressure, pulse, 3-lead ECG, heart rate variability, bodyweight, oxygen saturation, blood glucose, natriuretic peptides). Others obtained from invasive devices are recorded automatically (eg, impedance, incidence of arrhythmias, pulmonary and left atrial pressure.38
TELEHEALTHCARE IN HEART FAILUREHeart failure is associated with substantial morbidity, mortality, and healthcare costs.34,39 Patients are often followed closely in heart failure clinics but admittance to a cardiology department is frequently required, and the 30-day readmission rate is high.40 Telehealthcare may be a solution, providing a way to identify and monitor subclinical congestion. The earlier identification and treatment of congestion together with improved coordination of care may prevent hospitalization.
Ideally, contact between a patient and a healthcare provider is established with technology enabling the two parties to see and hear each other via a microphone and a computer screen over large distances using a telephone or satellite connection. One device is installed in the patient's home and one at the heart failure clinic. Preferably the patient's device is portable and capable of both videoconference and data transmission, and can be managed by the healthcare professional using a remote control.
According to individual arrangements patients could be instructed to record blood pressure, heart rhythm and weight, and give a subjective description of symptoms every morning, and then transmit data daily, just prior to or during a scheduled remote follow-up. Data can then be reviewed just before the videoconference with the patient, so treatment can be altered if needed. It is important that the healthcare professional be empowered to change medication regimens so delay is diminished and the correct treatment established in a timely manner.
Several advantages of the use of telehealthcare in heart failure have been reported.36,41 Patients get a better understanding of their disease by being responsible for monitoring and being involved in some self-managing, which enhances patient education and empowerment. This supports adherence to the planned treatment, but also ensures preventive care with early detection of disease exacerbations and timely management. Travel and waiting time is reduced or even abolished for the patient, and quality of life improves. Additionally, patients are at home in comfortable and safe surroundings and still in close contact with the hospital. An advantage for the hospital is that the technology leaves room for new and unstable patients, and long admittances to hospital may be prevented.
However, the effectiveness of telehealthcare by reducing morbidity and mortality in heart failure patients has not been established because results have been conflicting. Accordingly, 2 metaanalyses42,43 have suggested that telehealthcare reduced morbidity and mortality but 2 prospective randomized clinical trials not including the metaanalyses did not support those findings.44,45 A possible explanation for this difference may be that the metaanalyses combined many small dissimilar studies of patients with varying risk profiles followed for different durations of time and with differences between studies in the intervention used. A newly published prospective randomized trial of 3230 patients with either obstructive pulmonary disease or heart failure showed that telehealthcare was associated with lower mortality, emergency admission rates, and shorter length of admission.46 An ongoing Danish study randomizes chronic heart failure patients to either traditional treatment in outpatient clinics or monitoring by telehealthcare. Another part of the study randomizes patients admitted with acute heart failure to traditional treatment in hospital versus early discharge and daily contact with the hospital via telehealthcare. The hypothesis is that at-home treatment of heart failure patients is feasible, effective, and safe, and thereby releases resources to other patient groups (personal communication).
In the studies mentioned above telehealthcare was based on noninvasive data, but other studies have investigated the management of heart failure patients using data from implantable devices. An increase in pulmonary vascular congestion is reflected by increased impedance, and thus intrathoracic impedance monitoring may be a valuable tool in the management of heart failure in patients, such as those with an implantable cardioverter-defibrillator (ICD)47. This is supported by a study that found significantly greater sensitivity to predict worsening heart failure events for intrathoracic impedance monitoring compared to acute weight increase.48 Likewise, left ventricular filling pressures and pulmonary artery pressures are correlated with clinical congestion, functional limitation, and poor prognosis in heart failure patients.49 Since the intracardiac and pulmonary artery pressures increase several days to weeks before the onset of clinical symptoms of congestion, close monitoring may provide early warning and assist appropriate management, including changes in medication. Abraham et al.50 used a wireless implantable hemodynamic monitoring system to measure pulmonary artery pressure, and reported that the use of the system significantly reduced the duration of hospital stays and the rate of hospital admissions related to heart failure, the latter by 30%.
In a study that monitored left atrial pressure, this monitoring was associated with reduced risk of acute decompensation and death.51 Additional randomized clinical trials using third- or fourth-generation telemedical systems that combine noninvasive and invasive variables with daily monitoring and management are needed to fully clarity the value of telehealthcare in cardiology.
TELEHEALTHCARE AND IMPLANTABLE DEVICESThe number of patients with pacemakers and ICDs has increased tremendously over the years as indications have broadened to include more patient groups.35 According to guidelines, patients with a pacemaker or an ICD need a follow-up at 3-12 months and 3-6 months, respectively.52 Usually the follow-up visits include both an evaluation of the device function (including battery status and capture thresholds) and a clinical examination, which may result in reprogramming of the device or changes in medical treatment. These time-consuming visits can be a burden to the patient, as they often require long transportation, waiting time, and time off work. For the outpatient clinic, the many visits require both space and a large staff because of the workload associated with every visit.
Remote monitoring and remote follow-up by the use of telehealthcare offers an alternative management strategy.53 Preferably, the system should be able to automatically transmit data stored in the device to the outpatient clinic using the wireless global system for mobile communication network or a landline. Automatic wireless data transmission requires a pacemaker or ICD equipped with a micro-antenna for communication with a transmitter located close to the patient. This setup diminishes the need for patient compliance and increases the frequency of transmissions.
Several companies provide systems for automatic wireless data transmission.21 Data stored in the device's memory (eg, battery voltage, lead characteristics, arrhythmias, alerts) can be transmitted. The alerts may be based on a change in device performance (battery status, lead impedance), programming (disabling of ventricular fibrillation therapy, insufficient safety margins for sensing or capture), or medical data (arrhythmias, indication of lung fluid accumulation). Events that trigger an alert can be defined according to the individual patient. Data is usually transmitted to a central database, where it is processed and made available to the physician on a secure webpage. Additional notification by e-mail, SMS, fax, or phone messages may be valuable when critical data are available for consultation.53
Many pacemakers and ICDs can automatically run tests like battery status, lead impedances, sensing and capture thresholds that used to be manually performed at the outpatient clinic.53 Both scheduled follow-up, with transmission of pre-defined data, and unscheduled monitoring can then be performed. Daily monitoring allows transmission of data with any predefined alerts to a physician. Consequently, remote monitoring may improve patient safety and quality of care. Depending on the need for reprogramming, clinic follow-ups are still needed because due to safety issues no system yet allows remote device programming.
The safety and clinical benefits conferred by telehealthcare in patients with implantable devices have been validated in several studies.54–56 In the COMPAS trial,54 long-term remote monitoring of pacemaker recipients was a safe substitute for conventional follow-ups, decreased the number of ambulatory visits, and enabled early detection of important clinical and device-related adverse events. Another study showed that half of the regularly scheduled visits could be omitted by the use of remote monitoring, without impairing patient safety.55 Additionally, only 6% of patients undergo device reprogramming or admittance to hospital during clinic visits, and thus 94% of these visits might as well be executed by remote monitoring.56 Remote monitoring could diagnose 99% of arrhythmia- or device-related problems in patients with an ICD, when combined with a clinical follow-up. In addition to fewer scheduled clinic visits, unscheduled visits following an ICD shock might be prevented by remote follow-up; after such an event the patient may perform manual upload of data, transmitted to the healthcare provider for immediate determination of whether the shock was appropriate or not, and whether device reprogramming or medical modifications are needed.57 Finally, several studies have demonstrated that remote monitoring reduces the time to event recognition and diagnosis and the time from the event to a clinical decision for the individual patient.58,59
Remote monitoring is also advantageous in patients with paroxystic atrial fibrillation because alerts could identify the start-point of atrial fibrillation, resulting in an unscheduled follow-up either in office or by phone, and appropriate interventions (eg, antiarrhythmic medication, anticoagulation or antiplatelet medication, external cardioversion, device reprogramming).60 In general, satisfaction is high and both patient and healthcare personals prefer remote follow-up over in-clinic visits.61,62
FUTURE TELEMEDICINE IN CARDIOLOGYTelemedicine has revolutionized modern cardiology and provided the opportunity for consultations between doctors separated by long distances, even between continents. In the acute phase of heart disease, telemedicine has helped to deliver optimal care faster and in chronic disease consultation between doctor and patient decreases the need for outpatient visits. As these systems are further improved, so are patient outcomes. In the future, it is likely that smaller implantable devices will become available to provide wireless feedback to patients and doctors alike via interfaces that connect through computers or mobile devices.
CONFLICTS OF INTERESTNone declared.