Heart failure is the end-stage of many cardiovascular diseases—such as acute myocardial infarction—and remains one of the most appealing challenges for regenerative medicine because of its high incidence and prevalence. Over the last 20 years, cardiomyoplasty, based on the isolated administration of cells with regenerative capacity, has been the focal point of most studies aimed at regenerating the heart. Although this therapy has proved feasible in the clinical setting, the degree of infarcted myocardium regenerated and of improved cardiac function are at best modest. Hence, tissue engineering has emerged as a novel technology using cells with regenerative capacity, biological and/or synthetic materials, growth, proangiogenic and differentiation factors, and online registry systems, to induce the regeneration of whole organs or locally damaged tissue. The next step, seen recently in pioneering animal studies, is de novo generation of bioartificial hearts by decellularization and preservation of supporting structures for their subsequent repopulation with new contractile, vascular muscle tissue. Ultimately, this new approach would entail transplantation of the “rebuilt” heart, reestablishing cardiac function in the recipient.
Keywords
.
INTRODUCTIONHeart failure is the end-stage of many cardiovascular diseases—such as acute myocardial infarction—and remains one of the most appealing challenges for regenerative medicine because of its high incidence and prevalence.1,2 Patients with progressive cardiac dysfunction show a high risk of sudden death and, despite substantial advances in recent years, cardiac function is only fully reestablished after heart transplantation (although its use is limited by the scarcity of donors and the possibility of complications). Acute myocardial infarction occurs when the blood supply to the heart is interrupted, causing irreversible myocardial ischemia, loss of cardiac muscle cells (cardiomyocytes), and formation of a noncontractile scar.3 Consequently, there is a need to develop therapeutic strategies that can promote rapid reconstruction of the affected tissue and efficient renewal of its contractile capacity.
Over the last 20 years, cardiac cell therapy (cardiomyoplasty), based on the isolated administration of cells with regenerative capacity, has been the focal point of studies seeking to regenerate the heart.4–7 The results of clinical trials have shown that the procedure is safe, although the benefits in terms of increased ejection fraction are modest. At the time of writing, several studies are trying to confirm the clinical benefit of cell therapy.
Attention has recently focused on new procedures based on combining cells with regenerative capacity, proangiogenic growth factors, biological matrices, biocompatible synthetic polymers and online registry systems that use bioimplants. Together, these advanced techniques are known as tissue engineering.8–12 One step further, proposed in pioneering studies in animal models, would be to generate de novo bioartificial hearts, decellularizing them and preserving the construct structure to repopulate them with new contractile, vascular, muscle tissue.13 This novel approach would ultimately lead to transplantation of the “reconstructed” heart to reestablish cardiac function in the recipient.
The present update analyzes the status of tissue engineering and its advantages and disadvantages. An overview of these techniques suggests a highly promising future in the struggle to recover the dysfunctional myocardium.
CELLULAR CARDIOMYOPLASTYCardiomyoplasty aims to restore the damaged myocardium through isolated implantation of cardiomyogenic and/or angiogenic stem cells in the dysfunctional ventricle.5 The key issues surrounding this therapeutic strategy are the choice of cell type and the most appropriate route of delivery.
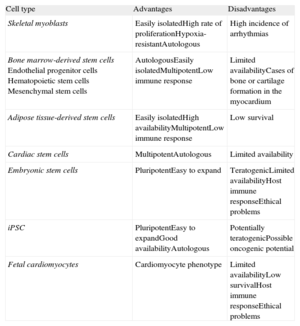
Cells With Cardiac Regeneration PotentialAn ideal source of cells would: a) expand in vitro on a large scale; b) integrate with damaged tissue, and c) differentiate into new cardiomyocytes electromechanically coupled with the host tissue (Table).
Advantages and Disadvantages of Implanted Cells
| Cell type | Advantages | Disadvantages |
| Skeletal myoblasts | Easily isolatedHigh rate of proliferationHypoxia-resistantAutologous | High incidence of arrhythmias |
| Bone marrow-derived stem cellsEndothelial progenitor cellsHematopoietic stem cellsMesenchymal stem cells | AutologousEasily isolatedMultipotentLow immune response | Limited availabilityCases of bone or cartilage formation in the myocardium |
| Adipose tissue-derived stem cells | Easily isolatedHigh availabilityMultipotentLow immune response | Low survival |
| Cardiac stem cells | MultipotentAutologous | Limited availability |
| Embryonic stem cells | PluripotentEasy to expand | TeratogenicLimited availabilityHost immune responseEthical problems |
| iPSC | PluripotentEasy to expandGood availabilityAutologous | Potentially teratogenicPossible oncogenic potential |
| Fetal cardiomyocytes | Cardiomyocyte phenotype | Limited availabilityLow survivalHost immune responseEthical problems |
iPSC, induced pluripotent stem cells.
In this context, adult stem cells have been obtained from bone marrow, adipose tissue, skeletal muscle, dental pulp, peripheral blood, amniotic fluid, and synovial fluid.14 More specifically, in the field of cardiac regeneration, skeletal myoblasts have been implanted because they are easily isolated, have a high rate of proliferation and are hypoxia-resistant.15,16 Similarly, different cell populations residing in bone marrow have been tested because of their great plasticity toward cells of cardiogenic and endothelial lineage17: endothelial progenitor cells,18,19hematopoietic stem cells4 and mesenchymal stem cells.20 As an alternative source of mesenchymal stem cells, subcutaneous adipose tissue enables us to obtain a large number of cells,21 which have been applied in clinical trials with attractive results.22 Moreover, progenitor cells with high cardiomyogenic and vasculogenic potential have been identified in the adipose tissue surrounding the heart. Implanting these cells improves heart function and reduces infarction size in murine and rat models.7
It was long thought that mammal hearts lacked any self-regenerating capacity. This view has been discounted, partly due to the discovery of cardiac stem cells, residing in the heart, which are self-replicating and capable of generating cardiomyocytes, endothelial cells and cardiac fibroblasts.23,24 These cells have been identified and isolated using the Sca-1, c-kit, ABCG2 and Islet-1 markers23–25 and from the formation of cardiospheres from myocardial explantations.26 These findings have given rise to strategies based on activating these cells with growth factors that favor their survival and cellular migration.26,27 However, several studies have shown that cardiac stem cells implanted in animal models of myocardial infarction fuse with the cardiomyocytes of the recipient.23,28
As an alternative, embryonic stem cells have been tested because of their strong capacity for expansion and subsequent differentiation into cardiomyocytes, endothelial cells and cardiac fibroblasts.29,30 To avoid using this type of nonautologous cell and the consequent need for immunosuppression therapy, induced pluripotent stem cells have been developed from somatic human tissue.31,32 Like embryonic stem cells, induced pluripotent stem cells have limited replication and ample capacity for differentiation. Cardiomyocytes of fetal origin are another cell type that has been used. These cells are capable of surviving, proliferating and forming intercalary discs with host myocardial tissue.33–35
Routes of Cell DeliveryAnother decisive issue in optimizing cardiomyoplasty is the route of cell delivery. Intramyocardial injection has been tested using the epicardial approach via sternotomy,36 the endomyocardial route,37 and the intracoronary route.38 The intracoronary route is widely recommended as it offers higher indices of intramyocardial cellular retention39,40; however, this retention does not exceed 10% and most cells delivered nest in other organs or die.41 Independently of the route of delivery used, cardiomyoplasty has shown modest improvements in cardiac function and limited survival of cells implanted in the fibrous myocardium.
LimitationsStudies in animal models based on the above-mentioned use of cells and routes of delivery indicate that cardiomyoplasty is a feasible, safe and beneficial technique. Nonetheless, although a viable therapy in the clinical setting, the extent to which infarcted myocardium is regenerated and cardiac function improved is highly limited. Basically, cells implanted under mechanical forces show poor survival, and recipient tissue hypoxia prevents them from providing any therapeutic effect.42–45 Moreover, very few cells differentiate into new cardiomyocytes and, because they lack any electromechanical properties, the regenerated muscle tissue is dysfunctional. For example, the high incidence of arrhythmias due to the lack of electromechanical coupling has undermined the use of myoblasts to treat patients with cardiac dysfunction.16,46 Furthermore, the status of indifferentiation of embryonic stem cells generates their uncontrolled proliferation, giving rise to the formation of teratomas,47 whereas obtaining induced pluripotent stem cells entails using viral infections that could also promote unwanted oncogenic activity.31,32
In light of the above, new therapeutic strategies, such as tissue engineering, are being developed and are reviewed in detail in the following pages.
CARDIAC TISSUE ENGINEERING: BIOPROTHESES FOR THE MYOCARDIUMCardiac tissue engineering is a complex new technology based on the use of combinations of cells with regenerative capacity, biological and/or synthetic materials, growth factors, differentiation factors and proangiogenic factors, and online registry or monitoring systems to induce the regeneration of an organ or damaged tissue. The principle objectives of cardiac tissue engineering are to generate cell matrices, establish electromechanical coupling, and validate stable contractile function and functional vascularization.9
The heart has dynamic functional properties that require a sophisticated tissue architecture with cellular components and specialized extracellular components.48 A key characteristic enabling the heart to activate circulation and satisfy its varied demands during rest and exercise resides in the asymmetrical architecture of the helicoidal myocardial band.49 Recently, the electromechanical role of the extracellular matrix has been found to be more significant than previously thought.50 The ideal artificial cardiac tissue would reproduce these structural, mechanical and electrophysiological properties to keep transplanted cells viable, and would stimulate vasculogenesis in the implanted tissue itself. Therefore, the use of natural or synthetic polymeric materials and biological matrices, applied directly on the infarcted area or used as a construct matrix, constitutes an alternative to cell cardiomyoplasty.
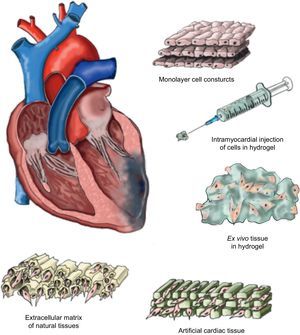
Matrix TypesThe use of a matrix—not necessarily biological in origin but biocompatible—means that the delivered cells can access a stable support structure that facilitates the correct localization and retention near the tissue requiring their therapeutic effect. The structure of these matrices should fulfill certain “practical” requirements, such as maintaining a permanent flow of nutrients and oxygen between the cells available in their interior and the microenvironment surrounding them, and facilitating efficient migration and survival within the ischemic tissue. Moreover, the ideal matrix would be biodegradable, producing no toxic products, so that it could finally be replaced by viable new tissue. We will now summarize some—but inevitably not all—of the approaches studied to date in the context of cardiac tissue engineering (Fig. 1).
Cells cultured in temperature-sensitive polymer plaques facilitate the detachment of cell monolayers without the need for enzymatic intervention.51 Once complete, this construct adheres to the ischemic zone to favor intramyocardial implantation of cell monolayers. The formation of new blood vessels has been reported, as has functional improvement due to the joint implantation of various monolayers of adipose tissue-derived mesenchymal cells in a murine model of chronic myocardial infarction.52 Moreover, the use of overlaid neonatal cardiomyocyte monolayers has been shown to generate intercellular communication that activates contractile function and propagates signals within the construct.53 Furthermore, intercalation of endothelial cell monolayers favors the formation of new vessels in the ischemic zone. Despite the benefits observed through the use of cell monolayers, this strategy still lacks translational character, as obtaining a construct of similar characteristics that guarantees the same results in the human heart is inviable.
Intramyocardial Injection of Cells in HydrogelAnother approach is based on developing natural hydrogel types such as MatrigelTM (laminin, type IV collagen and heparan sulphate),54 collagen,55 or fibrin,56 in which the therapeutic cell population is embedded for subsequent intramyocardial injection. Although the effect of hydrogel on cellular retention has been positive,54–56 the injection pressure needed for its delivery is too great and causes high cell mortality, which notably diminishes its possible therapeutic effect. Similarly, the use of hydrogels made from natural materials entails having little control over their physiochemical properties and degradation. Moreover, hydrogels are difficult to sterilize and purify.57 As an alternative, synthetic hydrogels have been developed, such as polyethylene glycol, polylactic acid, polylactic acid-co-glycolic acid, polycaprolactone, polyacrilamide and polyurethane, which minimize these disadvantages.58 However, their cytotoxic potential is still being studied and the US Food and Drug Administration has only approved the use of polyethylene glycol, polylactic acid and polylactic acid-co-glycolic acid for clinical application. It has also been shown that matrix metalloproteinase-sensitive polyethylene glycol enables us to modulate the elasticity, biophysical and biochemical parameters involved in cardiomyogenic differentiation of implanted cells.59 An alternative method is to use hybrid natural/synthetic hydrogels that provide the advantages of both polymer types.58
Ex Vivo Formation of Hydrogel Cell TissueTo counter the disadvantages of intramyocardial hydrogel injections, an alternative based on the ex vivo creation of new tissue from cells with cardiovascular potential previously incorporated into hydrogel has been studied. Two recent studies report the in vitro contractile capacity of constructs composed of embryonic cardiomyocytes10 or neonatal rat cardiomyocytes.11 This strategy creates a 3-dimensional environment favoring intercellular communication and preventing anoikis60 (cell death due to the absence of intercellular communication) and enabling the cell constructs themselves to form an extracellular matrix.61 However, to validate optimal tissue development, these new constructs should clearly be submitted to mechanostimulation—otherwise the cardiomyocytes tend to die.62
Artificial Cardiac TissueThe microenvironment in which regenerative cells reside is decisive in maintaining their basic properties and function. In fact, microarray studies have confirmed that communication between cells depends, to a large extent, on their interactions with components of the extracellular matrix where they reside.63 Consequently, cardiac tissue engineering favors new alternative approaches based on the formation of functional 3-dimensional artificial cardiac tissue. For example, matrices have been developed with physiochemical properties very like the physiological extracellular matrix but based on natural materials, such as alginate64 or mixtures of collagen,10 or synthetic materials, such as polylactic acid65 or polyglycolic acid.66 The principle advantage is that these materials are highly malleable, which allow their form and size to be varied according to the needs of the individual recipient. The MAGNUM (Myocardial Assistance by Grafting a New Bioartificial Upgraded Myocardium) clinical trial used a type I collagen matrix large enough to completely cover the myocardial scar. MAGNUM compared isolated cardiomyoplasty with a combination of cardiomyoplasty and tissue engineering and concluded that this new alternative offers better results in terms of functional recovery and ventricular remodeling.67 The artificial cardiac tissue tested, however, does not match the extracellular cardiac matrix perfectly and the implanted cells only colonize the surface or a depth of up to a few micrometers.61 A European consortium (RECATABI, REgeneration of CArdiac Tissue Assisted by Bioactive Implants)68 has recently been created to develop a cardiac bioengineering platform to combine innovative biomaterials (an elastomeric skeleton and hydrogel-PuraMatrixTM) that will improve the delivery, survival and proliferation of implanted cells. The preliminary results show a certain degree of cardiomyogenic differentiation of implanted cells and vascular connections between constructs and the adjacent myocardium.
Extracellular Matrix Derived From Natural TissuesThe extracellular matrix is composed of functional and structural proteins such as collagen, elastin, laminin, fibronectin, proteoglycans and many other glycoproteins, combined and spatially organized by tissue type.69,70 This matrix is known to participate in many processes and cellular responses including proliferation, differentiation and migration.71 These properties make it potentially attractive as a support structure in cardiac tissue engineering techniques to implant regenerative cells that substitute the damaged myocardium. Moreover, the implanted extracellular matrix may be capable of substituting that of the damaged tissue, efficiently contributing to its regeneration. Extracellular matrix lamina have been successfully isolated from a variety of tissues including native cardiac valves,72–74 blood vessels,75,76 skin,77 nerves,78 skeletal muscle,79 tendons,80 ligaments,81 small intestinal submucosa,82 urinary bladder,83 and liver.84
To apply these lamina correctly, they should be separated from native tissue, decellularized and, often, disinfected, freeze-dried and sterilized.85 Clearly, this complex process could affect matrix integrity and architecture. The use of animal—particularly porcine—tissue as a source resolves the critical scarcity of human tissue for surgical applications.86
The ideal decellularization protocol for matrices is one that can selectively eliminate allogenic and xenogenic antigens, as well as all tissue cell and nuclear content while preserving its composition, physiological properties, the mechanical integrity of the extracellular matrix, and the vascular structure.87,88 This process combines specific physical, chemical and enzymatic treatments according to tissue type.79,83,89,90
Many studies have shown that implanting the extracellular matrix facilitates tissue remodeling in animal models and in the clinical context.83,89,91–97 Experimentally, colonization of extracellular matrices implanted by cells of both cardiomyogenic and endothelial lineage, thus avoiding ventricular remodeling, has been reported in animal models.98–101 In extracellular matrices of cardiac origin, the results suggest improved heart function with the cardiomyocytes present in the region of the implant.102 Recent publications have shown that using extracellular myocardial matrices and preserving their 3-dimensional architecture is a key alternative to facilitate the support and cellular differentiation needed to favor cardiac regeneration.13,103–109
Electromechanical Cellular Coupling and Contractile FunctionIn physiological conditions, mechanical stretching of cardiomyocytes is induced by electric cardiac signals and coupling between the electric pulse and cellular contractions, and is critical in developing the myocardium.14 Therefore, it is vitally important that cardiac tissue engineering techniques guarantee electromechanical cellular coupling and adequate contractile function within the constructs generated. To this end, structures have been designed with collagen and MatrigelTM in the form of an annulus colonized by neonatal rat cardiomyocytes and subjected to mechanical stimulation.10 After implantation into the ischemic rat myocardium, these annular structures remain autonomously contractile and are responsible for the increased thickness of the infarcted ventricle wall and improved heart function. Another approach is based on inducing synchronized contractions in the cellular matrix through electric stimulation.110,111 As a result, electric pulses give rise to substantial ultrastructural organization and cellular coupling of cardiomyocytes residing in the matrix. To balance this effect, these constructs contain allogenic cardiomyocytes and are too small for clinical application.
A study was recently started in a porcine model of acute myocardial infarction based on implanting a bioactive matrix with an online monitoring system. Using electric impedance, this model allows the progress of cardiac scarring to be determined in situ and the electrical changes due to this process to be assessed.112,113 This new approach will, in the future, enable noninvasive assessment of the functional improvements occurring after cell delivery through cardiac tissue engineering.
Functional VascularizationOne of the key objectives of cardiac tissue engineering is to promote vascularization phenomena within the bioartificial matrix that allow continuous diffusion of nutrients and oxygen toward the interior of the matrix. Such phenomena could subsequently favor the migration and incorporation of cells into the damaged myocardium. Several means of guaranteeing this process have been tested. For example, growth factors such as vascular endothelial growth factor114 or basic fibroblast growth factor115 have been included as part of the construct to stimulate vascular structure formation from the mesenchymal stem cells116 and/or endothelial progenitor cells117 of the construct, or mobilizing cytokines have been added to incorporate the recipient's own endothelial progenitor cells following implantation. On the whole, this approach promotes cellular infiltration and the formation of blood vessels with highly promising results.114–119 Other alternatives include the in vitro use of bioreactors to improve oxygen perfusion through the implanted cells inside the previously canalized matrix to facilitate endothelial progenitor cell migration toward the interior of the constructs generated.14
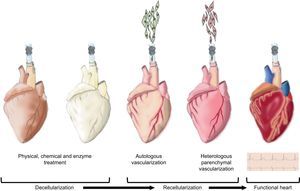
Neo-organogenesis: Bioartificial HeartThe above-mentioned decellularization studies with extracellular matrices provide conceptual proof that decellularized hearts can be obtained. To date, direct immersion decellularization processes have been sufficient to generate construct matrices from different cardiovascular tissues, including the valve wall,120 pericardium,121,122 and valvular valves.123,124 In contrast, it has been demonstrated that coronary perfusion with detergents is the most efficient means of decellularizing a whole heart. In 2008, cadaver rat hearts were successfully decellularized to obtain a complex extracellular cardiac matrix with preserved vascular tree, competent valves and intact atrial and ventricular geometry.13 These constructs are subsequently recellularized with endothelial cells (autologous vascularization) and neonatal cardiac cells (heterologous parenchymal recellularization) using coronary perfusion in a bioreactor simulating the cardiac physiology and favoring the maturation of the organ (Fig. 2). After 8 days of physiological incubation and electrical stimulation, macroscopic contractions were detected with a pump function equivalent to 2% of the adult heart or 25% of the heart function of a 16-week fetus.13
Neo-organogenesis: bioartificial heart. Schematic representation of the different stages in the decellularization and recellularization of a heart. Initially, physical, chemical and enzymatic decellularization is intended to preserve the extracellular cardiac matrix and vascular tree. Next, autologous vascularization is induced followed by heterologous parenchymal recellularization in order to finally generate a new functional heart.
More recent studies have reported decellularization of porcine hearts as a model that can be scaled to human proportions.105,109 One important aspect to consider in the application of whole organs or bioartificial matrices is the need for correct vascularization without which construct viability could be compromised. This problem is especially critical when matrix thickness is greater than the diffusion barrier (approximately 100μm), where the oxygen and nutrient supply is limited and cellular cytotoxic waste subproducts accumulate.103,125 Decellularization of whole hearts has led to the successful mimesis of vascularized myocardial tissue and its repopulation with cells through the vascular structures with a certain degree of preservation.13,105,109 Despite its success, this strategy can be limited with respect to the technology available for the large-scale expansion of cells—particularly cardiomyocytes—necessary to repopulate the entire organ.103,126 Much remains to be done before a bioartificial heart is available for use in humans. However, in Spain, a pioneering research group in the field is developing a bioartificial heart, as first conceptual proof, by decellularizing human hearts and subsequently recellularizing them with human mesenchymal cells and murine cardiomyocytes.127
CONCLUSIONS AND PROSPECTS FOR THE FUTUREAlthough the results of the above-mentioned studies are clearly promising and many show a noteworthy improvement in cardiac contractile function, many issues remain to be defined. At the time of writing, the major disadvantage of most tissue engineering studies lies in the difficulty of extrapolating the mouse/rat animal model to the porcine model and, consequently, the clinical context. The size of the human heart makes these approaches inviable, both due to matrix dimensions (10-50cm2 and several millimeters thick14) and the limited number of cells that can actually be implanted.
Faced with this limitation, our research group has designed a new surgical technique based on transposing an autologous pedicle of pericardial origin onto the ischemic myocardial surface. This new proposal offers highly promising results in a preclinical porcine model of myocardial infarction given that the fatty pedicle establishes vascular connections in the infarcted myocardium128,129 and consequently guarantees improved cardiac function in terms of ejection fraction and cardiac volumes.128 Currently, we are prospectively enrolling and randomizing patients (Clinicaltrials.gov NCT01473433) to determine the safety and effectiveness of this new surgical approach.130 In the clinical context, few trials have been based on the application of cardiac tissue engineering. Two current clinical trials are investigating the intramyocardial injection of alginate (NCT01311791) or intracoronary administration of sodium alginate combined with calcium gluconate (NCT01226563) in order to generate a new extracellular matrix in the myocardium for the resident cardiac progenitors to migrate and repopulate the cardiac scar.
Despite numerous advances in cardiac tissue engineering, many questions remain hidden, which are crucial to the complete reestablishment of cardiac function and ischemic myocardium vascularization. Firstly, the key is to determine which cell type (adult cells, embryonic cells, or induced pluripotent stem cells; autologous or heterologous) is best-suited to obtaining tissue regeneration. Secondly, we need to define which matrices (natural or synthetic hydrogels, collagen, polylactic acid, polylactic acid-co-glycolic acid, extracellular matrix) are best able to nest the cell population and favor its retention and proliferation. Thirdly, the existence of electromechanical cell coupling within the matrix and the construct with the tissue under repair is fundamental to restoring heart function. Finally, vascularization of the construct will determine its own viability and integration with the recipient tissue, in addition to becoming the blood supply required to reverse the myocardial ischemia.
FUNDINGRed de Terapia Celular - TerCel (RD12/0019/0029), Red Cardiovascular (RD12/0042/0047), European Comission 7th Framework Programme (RECATABI, NMP3-SL-2009-229239), Ministerio de Educación y Ciencia (SAF2011-30067-C02-01), and Fundació Marató TV3 (122232).
CONFLICTS OF INTERESTNone declared.