Infective endocarditis is an uncommon disease, with an estimated incidence of 3.1 to 3.7 episodes per 100 000 inhabitants/year. The incidence is highest in elderly people. The microorganisms most frequently isolated in infective endocarditis are staphylococci and streptococci. In the last few decades, the spectrum of heart diseases predisposing to infective endocarditis has changed, since degenerative heart disease is the most common valve disease, and there are an increasing number of infective endocarditis patients without previously known valve disease. In addition, up to one-third of infective endocarditis patients become infected through contact with the health system. These patients are more frail, which leads to higher in-hospital mortality. As a result of substantial epidemiological changes, few cases of infective endocarditis can be prevented by antibiotic prophylaxis. Despite advances in medical and surgical treatment, in-hospital mortality among infective endocarditis patients is high. Nevertheless, there is room for improvement in reducing the rate of nosocomial bacteremia, the prompt diagnosis of infective endocarditis in at-risk patients, and the early identification of patients with a highest risk of complications, as well as in the creation of multidisciplinary teams for the management of this disease.
Keywords
.
INTRODUCTIONIn 2009, the most recent European Society of Cardiology guidelines defined infective endocarditis (IE) as an unusual disease for 3 reasons. First, in the last 30 years, the incidence of this disease and its associated mortality have remained unchanged despite clear advances in diagnosis and treatment. Second, IE is a highly heterogeneous entity with a wide spectrum of clinical manifestations that depend on the patient's underlying disease, the infecting microorganism, and the presence of local and remote complications. Hence, the management of these patients requires a multidisciplinary approach. Thirdly, given the lack of randomized studies, treatment guidelines are frequently based on expert opinions.1
Over the last few decades, the epidemiology of IE has changed in industrialized countries.2 The population at risk of infection are no longer young people with known rheumatic valvular heart disease and are now elderly people with no apparent valve disease.3 In addition, the mechanism of infection has also altered; a history of dental intervention before the onset symptoms has become anecdotal, while a substantial percentage of patients are infected by in-hospital procedures.4
Scientific publications on IE by Spanish hospitals in the last 20 years (1994-2013) has been considerable and continues to increase both in terms of the number and quality of the studies, allowing the current epidemiology of IE in Spain to be determined with considerable accuracy.
INCIDENCE OF INFECTIVE ENDOCARDITISNo population-wide studies of IE in Spain have been published, and consequently the national incidence of this disease is unknown. However, it is not unreasonable to assume that the situation should differ little from that of France, where population-wide observational studies are regularly conducted. These studies show that the incidence of IE is low but is slowly increasing: the age- and sex-standardized estimate was 2.4 episodes per 100 000 inhabitants/year in 1991,5 3.1 in 1999 (95% confidence interval [95%CI], 2.8-3.5),6 and 3.4 in 2008 (95%CI, 3.1-3.7).7 Although these figures describe the incidence in the general population, it is highest among men aged 75-79 years (19.4 episodes per 100 000 inhabitants/year).7
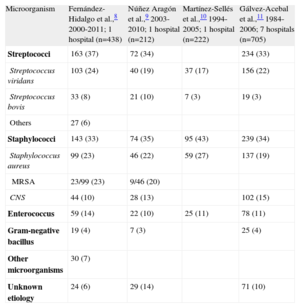
ETIOLOGYTable 1 shows the causes of IE in 4 contemporary Spanish series.8–11 Streptococci and staphylococci species are the 2 most frequent groups of causal microorganisms (approximately two-thirds of all episodes), followed by Enterococcus species, which cause approximately 10% of all cases.
Etiology of Infective Endocarditis in Different Spanish Series
| Microorganism | Fernández-Hidalgo et al.,8 2000-2011; 1 hospital (n=438) | Núñez Aragón et al.,9 2003-2010; 1 hospital (n=212) | Martínez-Sellés et al.,10 1994-2005; 1 hospital (n=222) | Gálvez-Acebal et al.,11 1984-2006; 7 hospitals (n=705) |
| Streptococci | 163 (37) | 72 (34) | 234 (33) | |
| Streptococcus viridans | 103 (24) | 40 (19) | 37 (17) | 156 (22) |
| Streptococcus bovis | 33 (8) | 21 (10) | 7 (3) | 19 (3) |
| Others | 27 (6) | |||
| Staphylococci | 143 (33) | 74 (35) | 95 (43) | 239 (34) |
| Staphylococcus aureus | 99 (23) | 46 (22) | 59 (27) | 137 (19) |
| MRSA | 23/99 (23) | 9/46 (20) | ||
| CNS | 44 (10) | 28 (13) | 102 (15) | |
| Enterococcus | 59 (14) | 22 (10) | 25 (11) | 78 (11) |
| Gram-negative bacillus | 19 (4) | 7 (3) | 25 (4) | |
| Other microorganisms | 30 (7) | |||
| Unknown etiology | 24 (6) | 29 (14) | 71 (10) |
CNS, coagulase-negative Staphylococcus; MRSA, methicillin-resistant Staphylococcus aureus.
Data are expressed as no. (%).
Because of the marked epidemiologic changes in IE in recent years,2 infection due to methicillin-resistant Staphylococcus aureus strains is increasingly frequent, hampering the medical treatment of these patients.12 Furthermore, population aging is increasing the number of infections due to bovis13 and Enterococcus.14 Finally, although Q fever IE is infrequent (3% of all cases), Spanish groups have made a noteworthy contribution to our understanding of this entity.15
CARDIAC RISK FACTORSThe spectrum of heart diseases predisposing patients to endocarditis has changed in the last few decades. Evidence of these changes in Europe was reported in the Euro Heart Survey,16 which found that degenerative heart diseases were the most frequent predisposing valvular heart conditions and that, in contrast, the number of patients with endocarditis and no previously known heart disease had increased considerably. In Spain, several studies have shown similar results. Castillo et al.3 reported 228 patients studied between 1987 and 2009: the incidence of predisposing rheumatic valvular heart disease and congenital heart diseases fell significantly, mitral valve prolapse was the most common heart disease, and the proportion of patients with no predisposing heart disease increased significantly (from 25% to 57% in recent years). The incidence of late prosthetic endocarditis has increased more than that of early prosthetic endocarditis.17 This trend was confirmed by a Spanish study indicating a fall in early prosthetic endocarditis from 0.94% between 1970 and 1986 to 0.34% between 1987 and 2003.18 However, Alonso-Valle et al.19 report no change in the incidence of early and late prosthetic endocarditis between 1986 and 2005. The incidence of pacemaker endocarditis and other device endocarditis has increased markedly,20 representing approximately 3% of cases in the series from Hospital Vall d’Hebron.
NON-CARDIAC RISK FACTORSHealth Care-associated InfectionsAs stated earlier, a substantial percentage of patients with IE acquire the infection through close contact with the health care system (≤35%, depending on the series).4,9,21 The term health care-associated infective endocarditis (HAIE) includes nosocomial IE, acquired in-hospital, and nosohusial IE, acquired as a consequence of ambulatory interventions (eg, hemodialysis, genitourinary examination, or cardiac catheterization).
Despite the heterogeneity of HAIE studies, all concur that the most frequent risk factor for contracting IE is vascular catheter-related bacteremia (in ≤63% of cases), followed by urological interventions (≤14%).
Patients with HAIE are more frail than those with community-acquired IE: their mean age is >8 years older; their baseline status is worse, as indicated by the Charlson index22 and the individual percentage of diabetes mellitus, chronic kidney failure or neoplasms; and they present more staphylococcal and enterococcal infections and higher in-hospital mortality. In fact, HAIE is an independent factor of in-hospital and accumulated mortality at 1 year.4,9 Hence, antiseptic measures should be rigorously applied before all invasive procedure.
Among patients with HAIE, an important subgroup consists of patients under renal replacement therapy using hemodialysis, who, in general series, represent ≤6% of all patients with IE.4,11,23 Among these, predisposing factors are the immunodeficiency associated with kidney disease, valvular calcification due to calcium and phosphorus metabolism and particularly the use of continuous vascular access. Specifically, the relative risk of bacteremia is 7.6 in patients with catheters vs 1.3 in those with fistulas.24 The real incidence of IE in this population is difficult to determine, since vascular catheter-related bacteremia is frequent, echocardiographic studies are not systematically conducted for each bacteremia episode and, even if they were, valvular calcifications can hamper image interpretation. In patients under hemodialysis, the incidence of IE is up to 60 times higher than in the general population,6,25 and in-hospital mortality is also higher.23
AgeThe incidence of IE is highest in elderly people,7 because they have a higher prevalence of predisposing heart disease and genitourinary and gastrointestinal infections than the younger population.26 Consequently, older persons have closer contact with the health care system and account for a higher proportion of patients with HAIE.
Over the last 20 years, the median age of patients with IE at Hospital Vall d’Hebron has increased by 15 years8,27 and currently stands at 66 years. With age, there is an increase in the percentage of enterococcal and S. bovis infections26 and a decrease in symptomatic peripheral embolisms and immune phenomena. Although mortality is clearly higher in >65 year-olds than in younger patients,28 no consensus exists on whether age is an independent risk factor of death, as several studies have shown widely differing results. Similarly, there is no agreement on whether or not surgical mortality is greater in this group of patients.26,29,30
Among patients aged >65 years, octogenarians have more streptococcal infections and fewer vegetations, undergo valvular replacement surgery less often, and have lower in-hospital mortality.30
Cirrhosis of the LiverIn Spanish cohorts, the prevalence of chronic liver disease in patients with IE ranges from 5% to 17%,11,31 whereas that of cirrhosis of the liver is ≤10%.32
Cirrhosis predisposes to the development of concomitant infections due to associated immunosuppression, ambulatory invasive procedures, and frequent hospitalization. In fact, one recent series reported that approximately 40% of patients with cirrhosis had one HAIE vs <20% of the rest of the population with IE.32 The same study reported that patients with cirrhosis were a mean 10 years younger than patients without liver disease; the most frequently isolated microorganism was S. aureus (25%), followed by beta-hemolytic Streptococcus (20%), and crude mortality was 51% (17% among Child class A patients and approximately 75% in Child class B and C patients). These figures have been corroborated in a multicenter study,11 and caution should be exercised when indicating surgery in these patients, especially those with advanced liver disease.
Intravenous Drug UsersOne of the most important changes in the epidemiology of IE in Spain has been the fall in the percentage of IE in intravenous drug users. This group represented a large proportion of infections in the 1980s and 1990s, accounting for <25% of all IE episodes in some series33, but is less important today. Evidence for this change can be seen in a contemporary IE series treated at our center (2000-2011) in which 6% were addicts, concentrated mainly in the early years of the study, whereas the current prevalence is <3%. A similar percentage (7%) was found in the an Andalusian cohort that included patients between 1984 and 2006.11
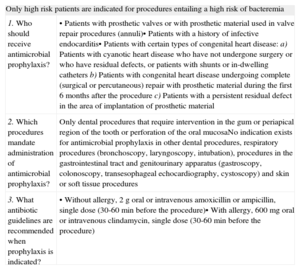
PROTECTIVE FACTORSAntibiotic ProphylaxisThe role of antibiotic prophylaxis in preventing endocarditis has been the focus of much debate in recent decades. The usefulness of this practice has been questioned and several national societies have reassessed the current literature and promoted new recommendations. The most extreme have been made in the guidelines of the National Institute for Clinical Excellence, which do not recommend antibiotic prophylaxis.34 The European Society of Cardiology guidelines, adopted by the Spanish Society of Cardiology,1 restrict the use of antibiotic prophylaxis to high-risk patients, ie, those with prosthetic valves, complex congenital heart disease, or a previous episode of endocarditis. The US guidelines are very similar.35 Recommendations on antibiotic prophylaxis of Hospital Vall d’Hebron are summarized in Table 2.
When and How to Use Antimicrobial Prophylaxis. Recommendations Followed at the Hospital Vall d’Hebron, Barcelona
| Only high risk patients are indicated for procedures entailing a high risk of bacteremia | |
| 1. Who should receive antimicrobial prophylaxis? | • Patients with prosthetic valves or with prosthetic material used in valve repair procedures (annuli)• Patients with a history of infective endocarditis• Patients with certain types of congenital heart disease:a) Patients with cyanotic heart disease who have not undergone surgery or who have residual defects, or patients with shunts or in-dwelling cathetersb) Patients with congenital heart disease undergoing complete (surgical or percutaneous) repair with prosthetic material during the first 6 months after the procedurec) Patients with a persistent residual defect in the area of implantation of prosthetic material |
| 2. Which procedures mandate administration of antimicrobial prophylaxis? | Only dental procedures that require intervention in the gum or periapical region of the tooth or perforation of the oral mucosaNo indication exists for antimicrobial prophylaxis in other dental procedures, respiratory procedures (bronchoscopy, laryngoscopy, intubation), procedures in the gastrointestinal tract and genitourinary apparatus (gastroscopy, colonoscopy, transesophageal echocardiography, cystoscopy) and skin or soft tissue procedures |
| 3. What antibiotic guidelines are recommended when prophylaxis is indicated? | • Without allergy, 2g oral or intravenous amoxicillin or ampicillin, single dose (30-60min before the procedure)• With allergy, 600mg oral or intravenous clindamycin, single dose (30-60min before the procedure) |
Clearly, these new recommendations change firmly-established medical practice. Furthermore, the level of dissemination and awareness of these recommendations among Spanish dentists is low.36 An article recently published in Revista Española de Cardiología defended the use of the European Society of Cardiology guidelines.37 However, doubts remain among clinical cardiologists and dentists, as reflected in Letters to the Editor.38 Some cardiologists and dentists fear that not applying prophylaxis in valvular heart disease patients could cause the incidence of endocarditis to rise again due to oral microorganisms of the oral cavity. However, a recent study appears to respond to this concern by showing that the incidence of endocarditis has not varied after the application of the new guidelines.39 As Falces and Miró37 suggest, the new guidelines should be disseminated among cardiologists and dentists and this dissemination should be coordinated as, for example, has happened in Catalonia where the societies of cardiology and dentistry have prepared a joint document.40
An important issue highlighted by the European guidelines is the crucial role of general preventative measures. These include patient education to ensure good oral hygiene, with regular dental check-ups, especially before valvular surgery. In addition, patients should be instructed on the risks of tattoos or piercings in nonsterile contexts.41 Finally, given the ever-growing increase in HAIE, it is vitally important to adhere strictly to antiseptic measures prior to any medical intervention, in particular before inserting venous access devices, especially in patients at risk of endocarditis. Similarly, at-risk patients should be instructed on the steps to be taken if they develop a fever. These measures would allow an earlier diagnosis and, consequently, improve prognosis (Table 3).
Instructions Given to Patients, After Discharge and After Valvular or Congenital Surgery, Valvular Heart Disease Unit, Hospital Vall d’Hebron, Barcelona
| Brush teeth daily |
| Have regular dental check-ups, at least once a year. A dental check-up is compulsory before elective valvular surgery |
| If you have a temperature >37°C for several days, take no antibiotics without previously consulting your physician and determining your chance of having a valve or prosthesis infection |
| If you suspect valve or prosthesis infection, attend the nearest hospital for a check-up |
| If your referral center is Hospital Vall d’Hebron, attend the infectious diseases day hospital (open Monday to Friday, 9.00 to 15.00). Outside of these times, report to the emergency room |
There has recently been speculation on the possible role of sex in the prognosis of IE. A study at Duke University42 indicated that women underwent fewer interventions than men and that mortality was higher among women, even though sex was not a predictor of mortality in multivariate analysis. The authors concluded that changes in therapeutic strategy and mortality were due to a higher number of comorbidities in women. In Spain, Castillo et al.43 also found that women with endocarditis underwent fewer interventions than men and that this finding was associated with greater mortality. Our group obtained similar results,44 with a lower surgery rate in the active phase in women and higher mortality, despite fewer complications, probably associated with a higher percentage of HAIE. More recently, Sevilla et al.45 reported a total of 621 cases of endocarditis and found that women were older and more frequently had diabetes mellitus and nosocomial disease; however, these authors found no differences between men and women in surgical interventions or mortality.
SURGERYWithout doubt, the most difficult issue in the management of patients with IE is deciding on the indications and timing of surgery. On the one hand, surgery would be indicated to prevent death or severe complications; on the other hand, surgery in the acute phase of the disease entails a high level of risk. Therefore, surgery should be performed in patients with clear indications and in the absence of comorbidities or severe complications that might seriously limit their chances of survival. In principle, age is not considered a contraindication to surgery, if required. In Spain, a large study has recently been published that reports surgical outcomes in octogenarian patients with IE.30 The European Society of Cardiology guidelines1 specify which patients should be considered for surgery and propose the optimal timing of interventions. Recently, the Society of Thoracic Surgeons have also published guidelines on the surgical management of endocarditis.46 However, despite this systematization, each case of endocarditis is a specific entity, involving multiple variables and should be; decision-making on surgery and its timing should therefore be individually tailored.
However, consensus appears to exist on the 3 main situations in which surgery should be seriously considered during the active phase of the infection: heart failure secondary to valvular dysfunction caused by the endocarditis, persistent signs and symptoms of sepsis despite correct antibiotic treatment (often accompanied by severe valvular destruction or perivalvular abscesses), and fear that systemic embolism might develop in patients with large vegetations.47
Another controversial issue is the urgency of surgery. Guidelines refer to indications for emergent, urgent (in the first week of treatment), and elective treatment. However, scarce data in the literature support this type of recommendation. San Román et al.48 have designed a multicenter clinical trial to determine the role of early surgery. In the absence of evidence in the medical literature, it is undoubtedly advisable to treat all patients with IE, if possible, in centers equipped for cardiac surgery or in a position to consult with specialized centers following diagnosis.49 Thus, the indication and timing of interventions will be decided by expert teams, if possible, before the infection leads to severe complications that substantially worsen prognosis.
SHORT- AND LONG-TERM PROGNOSISDespite undoubted advances in medical and surgical treatment, mortality from IE has remained practically unchanged over the last 20 years, which can be explained by the marked epidemiologic changes that have led to the current situation in which IE affects elderly people with a number of comorbidities.
In the Hospital Vall d’Hebron series of left valve IE treated between 2000 and 2011, in-hospital mortality was 29% (26% in native valve IE and 39% in prosthetic valve IE).8 The percentages were similar in the Andalusian series, with 30% crude mortality during the study period (26% between 1984 and 1995 and 32% between 1996 and 2006).11 Both studies guaranteed consecutive inclusion of all left valve IE episodes.
It is known that in-hospital or early mortality from IE largely depends on factors associated with the infection and its complications. Specifically, 30% of deaths are due to heart failure, 20% to neurologic complications, and 10% to uncontrolled infection.8 However, long-term mortality exclusively depends on the patient's underlying disease.
Numerous studies have assessed the multiple risk factors for death from IE. However, we cannot ignore the modifiable factors or attitudes that can alter the natural course of the disease. First, given the percentage of HAIE, antiseptic measures must be strictly followed before any invasive procedure, whether the presence of predisposing heart disease is known or not, because one-third of all IE episodes can be avoided.4 Second, diagnosis of these patients should be improved: those at-risk should be aware of their risk and attend their referral centers promptly if they develop fever of unknown etiology or constitutional syndrome. Third, after diagnosis, patients should be treated by multidisciplinary teams with experience in making decisions about this disease, given that they are seldom easy to make50,51 and that a multidisciplinary approach has clear and proven benefits.33,52 Finally, early identification of patients more likely to require surgery or at greater risk of death is feasible,53 which facilitates their early transfer to referral centers equipped for cardiac surgery.
AMBULATORY ANTIBIOTIC TREATMENTMedical treatment of IE is based on endovenous antibiotic administration, usually for 4 to 6 weeks, which entails lengthy hospitalization. However, it is known that the risk of complications in stable patients with good general health is minimal after the first 2 weeks of antibiotic therapy, allowing treatment to be completed out of hospital. This strategy can also be used in patients with a favorable clinical course after very early valve replacement. In addition to cutting costs, ambulatory administration of antibiotics improves patients’ quality of life. Consequently, a growing percentage of patients with IE have been included in programs for out-of-hospital administration of antibiotics through day hospitals and hospital at-home units.54,55 In our experience at the Hospital Vall d’Hebron, approximately one-third of patients who survive infection complete their antibiotic treatment in this context.
CONCLUSIONSIn Spain, an industrialized country, IE is currently an infrequent disease occurring in elderly people with no known predisposing heart disease and often as a consequence of close contact with the health care system. Despite continuous advances in the medical and surgical treatment of IE, mortality has remained stable. However, there is clear room for improvement in preventing episodes of catheter-related bacteremia, early diagnosis of infection, and identifying those at greater risk of needing surgery or of in-hospital mortality, as well as in creating multidisciplinary teams with experience in the medical and surgical management of this disease.
CONFLICTS OF INTERESTNone declared.