Keywords
INTRODUCTION
In recent years, mitral valve repair has become the surgical procedure of choice for mitral valve regurgitation (MR).1 When the aetiopathogenesis of MR is prolapse of one of the leaflets due to rupture or elongation of the chordae tendineae, the use of artificial chordae made of polytetrafluoroethylene (PTFE) has been offered as a safe and effective surgical alternative to for correction.2-6 The purpose of this study is to evaluate, through clinical and echocardiographic follow-up, the initial experience in our centre with the use of PTFE neochordae in mitral valve repair.
METHODS
Patients
This is a descriptive observational study of 21 patients who underwent mitral repair surgery with implantation of PTFE neochordae for MR at the Juan Canalejo University Hospital Complex (A Coruña) between April 2005 and July 2007. Mitral valve replacement was performed in 3 patients who initially underwent PTFE neochordae implantation due to suboptimal intraoperative transesophageal echocardiogram (TEE) results, so they were excluded from the study. Median surgical risk based on the logistical EuroSCORE was 3.95% (2.38%). The demographic and clinical data were obtained from the Apollo 32 database and from a review of the clinical history.
Surgical Technique
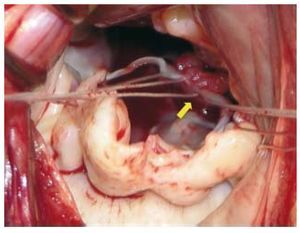
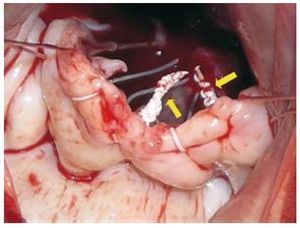
Cardiopulmonary bypass with bilateral cavae cannulation, hypothermia (32-34ºC) and myocardial protection with intermittent cold blood cardioplegia was used on all patients. Nine patients required additional associated surgical procedures (Table 1). For implantation of the PTFE neochordae, the suture is placed from the head of the papillary muscle to the free edge of the mitral valve leaflet (Figure 1) and the length is adjusted by using the non-prolapsed leaflet as a guide. Finally, the suture is tied with more than 10 knots towards the ventricular wall in such a way that it does not interfere with mitral valve coaptation (Figure 2). The median number of neochordae implanted per patient was 3 (range, 2-6). Mitral valve annuloplasty was also performed in all repairs with rigid, complete rings. In one case (patient 20), fusion of the P1-P2 scallops was performed as a complement to the repair.
Figure 1. Surgical image that shows implantation of the polytetrafluoroethylene neochordae in the postero-medial head of the papillary muscle (arrow).
Figure 2. Intraoperative image that illustrates the final disposition of the knots in all the polytetrafluoroethylene neochordae (arrows) oriented towards the ventricular wall in such a way that they do not interfere with mitral coaptation.
Follow-up
A transthoracic echocardiogram (TTE) was performed preoperatively and when the patients were discharged. Also, all patients underwent a baseline intraoperative TEE. All patients received anticoagulation in order to maintain an INR between 2 and 2.5 for 3 months or indefinitely for patients with atrial fibrillation or flutter. Clinical and echocardiographic follow-up was completed within a median of 9 (1-26) months. The level of residual MR was classified according to the mitral valve regurgitant orifice area (ROA.) Trivial MR was defined as ROA <2 cm2; mild MR was defined as ROA ≥2 and <4 cm2; moderate MR was defined as ROA ≥4 and <7 cm2 and severe MR was defined as ROA >7 cm2.
Statistical Analysis
The data were analyzed using Statistical Package for the Social Sciences (SPSS) version 13.0 for Windows XP. Descriptive statistics for continuous variables are expressed as the median (range) or the mean (standard deviation) as needed, while the qualitative variables are expressed as a percentage. The quantitative variables were analysed using the Wilcoxon range test, and McNemar's test was used for the qualitative variables, with a value of P<.05 being considered significant.
RESULTS
The patients' baseline characteristics are shown in Table 1. There was no hospital mortality. The median extracorporeal circulation time was 121 (80-137) minutes and the median ischaemia time was 92 (63-106) minutes. The median ICU stay was 2 (1-10) days and the median total postoperative hospital stay was 7 (5-17) days. Regarding postoperative complications, there were 3 cases of atrial fibrillation that returned to sinus rhythm with pharmacological treatment, 1 case of acute renal failure in a patient with chronic renal insufficiency and 1 case of type-1 postoperative neurological dysfunction that completely recovered. Two patients required inotropic support in the first 24 hours.
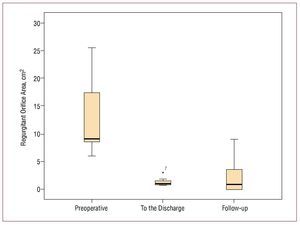
Regarding echocardiographic findings, the TTE at discharge revealed a statistically significant reduction in estimated MR by ROA (median, 1.10 [0.8-3.1] cm2; P=.001) compared to the preoperative study (median, 9.10 [6.1-25.4] cm2) (Figure 3). At the end of follow-up, the median ROA was 0.50 (0.9) cm2 (P=.001) and the median mitral area was 2.39 (1.82-3.3) cm2. Finally, the left ventricular ejection fraction (LVEF) (median, 63% [29%-77%]) and the pulmonary artery systolic pressure (PASP) (median, 39 [34-48] mm Hg) were not different compared to preoperative (LVEF: median, 62% [34%-85%]; P=.14; PASP: median, 40 [25-50] mm Hg; P=.38).
Figure 3. Box plot that represents the statistically significant reduction in the postoperative regurgitant orifice area (ROA) and in follow-up compared to preoperative.
All patients were found in NYHA functional class I-II at follow-up with the exception of one case that was at functional class III. Three patients in preoperative sinus rhythm had permanent atrial fibrillation. There were no haemorrhagic or thrombotic events associated with the anticoagulation treatment or the underlying cardiopathy, nor hospital readmissions due to heart failure. There was one death due to traumatic brain injury following an accident (patient 5).
DISCUSSION
Among the different techniques for mitral valve repair, implantation of PTFE neochordae has been shown to be a technique with low morbidity and mortality and short- and long-term efficacy.2-6 In this study, we analyse our initial experience with this surgical technique.
There was significant improvement clinically, and all of the patients were in NYHA functional class I-II after a median follow-up of 9 months with the exception of one patient who was class III. With regards to the echocardiographic findings, it should be pointed out that correction of MR was achieved in all patients (ROA <4 cm2), while 2 patients had moderate MR and 1 had severe-grade ROA at follow-up. There was no echocardiographic evidence of rupture of the neochordae in any of the cases. In the 2 patients with moderate MR at follow-up, the aetiology appears to be related to the length of the neochordae which may not be sufficient following a process of ventricular remodelling or dilation. In the case of severe MR at follow-up, detachment of the mitral ring in the anterior portion which caused MR was seen, with a ROA of 7.8 cm2. On the other hand, there were no signs of postoperative mitral valve stenosis. There was no case of hospital mortality or deaths due to cardiovascular aetiology during follow-up.
The performance of PTFE neochordae initially was studied in animals at the end of the 1980s7 and it was shown that within a few months, a fibrous sheath covering without calcifications was produced over the entire neochordae, with a macroscopic appearance that was very similar to native chordae. Similar histological findings in humans have been published,2,8,9 with a greater or lesser degree of fibrosis and occasionally even complete endothelialisation. On the other hand, several cases of dysfunction and rupture of PTFE neochordae due to excessive calcification have been reported.10,11 It is possible that certain underlying degenerative processes within the patient may play a role in the level and rapidity of onset of the histological patterns found in the dysfunctional neochordae.
Despite the limitations of this study with regard to the small sample size and the short-term follow-up, the results of our initial experience are encouraging and are in line with the previously published results.2-6 Therefore, a longer follow-up and a greater number of cases are needed in order to perform a more exhaustive evaluation of the surgical technique in our environment. Nevertheless, mitral valve repair surgery with implantation of PTFE neochordae has been offered as a safe and effective surgical alternative for MR in which the aetiopathogenesis is prolapse of one of the leaflets due to elongation or rupture of the native chordae tendineae.
Correspondence: Dr. F. Estévez Cid.
Servicio de Cirugía Cardíaca. Complejo Hospitalario Juan Canalejo.
Xubias de Arriba, 86. 15006 A Coruña. España.
E-mail: franciseste@yahoo.es
Received November 24, 2007.
Accepted for publication March 5, 2008.