Keywords
INTRODUCTION
Severe symptomatic aortic stenosis (AoS) has a poor prognosis, especially following the appearance of heart failure, with a life expectancy of less than 2 years without surgical repair.1-5 Aortic valve replacement (AoVR) is the only effective treatment recommended in clinical guidelines,6,7 but the surgical risk increases in the presence of left ventricular dysfunction.8 Theoretically, this ventricular dysfunction may improve following valve replacement, although this may not be the case if there is established myocardial damage (as a result of fibrosis or necrosis).8 There is still a paucity of data available on survival, changes in ventricular function, and long-term follow-up in patients undergoing AoVR for severe AoS with severely reduced systolic function. The aim of this study was to identify predictors of perioperative and long-term mortality and of recovery of ventricular function and functional class (FC) during follow-up.
METHODS
Study Population
We analyzed a retrospective cohort of 82 patients in whom surgical AoVR was performed for severe AoS between February 1996 and February 2008. Patients were included if valve replacement was performed exclusively with an aortic valve prosthesis (biologic or mechanical) and they had less than 40% ventricular function as measured by echocardiography or ventriculography. Thus, the study involved patients with severe AoS and ventricular dysfunction who underwent AoVR and included patients with or without a reduced transvalvular gradient prior to surgery. The following exclusion criteria were applied: a) valve replacement due to predominant aortic regurgitation or coronary heart disease with mild concommitant aortic valve damage; b) valve replacement in the context of type A aortic dissection with valve involvement or other disorders of the ascending aorta, along with those cases in which aortic annulus enlargement was also performed; c) repair or replacement of another heart valve; and d) mitral or tricuspid valve disease occurring as a result of rheumatic heart disease, endocarditis, or valve prolapse of any etiology.
Demographic, epidemiologic, clinical, electrocardiographic, and echocardiographic variables were analyzed. Coronary angiography was performed and the results analyzed. Data were also collected on the size of the valve prosthesis, morbidity and mortality in the immediate postoperative period or the period soon after surgery (up to 30 days), the requirement for repeat intervention, and morbidity and mortality during follow-up.
Standard American College of Cardiology/ American Heart Association definitions9 were used for the variables analyzed (cardiovascular risk factors, patient history, and postoperative complications).
Doppler Echocardiography
Echocardiography was performed prior to surgery using Acuson Sequoia (Siemens Co), Acuson Aspen (Siemens Inc), and VingMed (GE) equipment. Standard echocardiography included M-mode, 2-dimensional, and spectral and color Doppler with imaging of standard planes, including the long and short parasternal axes and apical 3-, 4-, and 5-chamber views. Echocardiography was performed prior to discharge in all patients who survived, and again in 33 of those during follow-up.
According to American Society of Echocardiography (ASE) guidelines,10 data were obtained on the aortic valve (maximum and mean gradient, valve area estimated by continuity equation, and assessment of aortic regurgitation), the mitral valve (morphology and function), and the tricuspid valve, as well as the presence and extent of left ventricular hypertrophy (defined on the basis of ventricular mass and wall thickness in M-mode in the parasternal long-axis view), size of the left atrium (anteroposterior diameter in the parasternal long-axis view and measurements in the apical 4-chamber view), systolic function, and pulmonary systolic pressure, when these could be estimated.
The severity of mitral regurgitation was estimated semiquantitatively by measuring the area of the regurgitant jet by color Doppler, pulsed Doppler trace, and pulmonary venous flow, as described in the ASE guidelines.11 All mild mitral regurgitation was included in the study meaning patients who underwent only AoVR. Those with severe concomitant mitral insufficiency also require treatment of the mitral valve and were not included.
Statistical Analysis
Continuous variables were expressed as means (SD) and categorical variables as percentages. Categorical variables were analyzed by c2 test or Fisher exact test when n<30 or when cells had an expected frequency <5. The Student t test was used for analysis of continuous variables. The Kolmogorov-Smirnov test was used to assess whether variables obeyed a normal distribution. A P value less than .05 was considered significant. A Cox logistic regression model was used for multivariate analysis to identify independent predictors of perioperative mortality and recovery of ventricular function. Variables were included in the model if they achieved a P value of less than .1 in the bivariate analysis or were recognized predictors of mortality in the literature. Odds ratios (OR) and 95% confidence intervals (CI) were calculated based on estimates obtained from the regression model. Kaplan-Meier survival analysis was performed and a log-rank test was used for comparison between groups.
RESULTS
Baseline Clinical Characteristics
The study included 82 patients with ventricular dysfunction who underwent exclusive surgical replacement of the aortic valve during the designated period and who met the inclusion criteria. The patients had a mean age of 69.63 (9.36) years and 74.4% were men. We calculated the mean EuroSCORE for our patient series and analyzed the variables included in this risk-stratification system that clearly influence mortality: chronic obstructive pulmonary disease (COPD) (26.8%), peripheral artery disease (8.8%), prior cerebrovascular accident (13.4%), requirement for repeat intervention (in our case there were no emergency repeat interventions), etc. A mean additive EuroSCORE of 13.4 (11.4) and a mean logistic EuroSCORE of 40.5 (34.3) were obtained. Thirteen patients (15.9%) had a severely reduced transvalvular gradient (<20 mm Hg). General baseline characteristics are shown in Table 1.
Surgical Results
A biologic prosthesis was implanted in 62.8% of patients (41 biological prostheses, of which 5 were stentless). Significant concomitant coronary lesions were present in 41.5%; aortocoronary bypass was performed in parallel in 77.1% of patients who had coronary lesions and was not performed for technical reasons (diffuse lesions, small vessel disease, or total occlusion with no viable tissue) in 22.9%.
Mortality in the Immediate Postoperative Period
Of the 82 patients in whom isolated AoVR was performed, 16 (19.5%) died during the postoperative period. The following postoperative complications were observed: low cardiac output in 37.8% of patients (defined as a cardiac index <2.2 L/min/m2 with pulmonary capillary pressure >15 mm Hg, ruling out hypovolemia as a cause, despite adequate control of heart rhythm, and in the absence of myocardial ischemia, valve dysfunction, or cardiac tamponade; pulmonary complications in 17,1% (acute respiratory stress, defined as severe, acute change in lung structure and function accompanied by increased vascular permeability that brings about pulmonary edema and is characterized by resistance to hypoxemia); cerebrovascular accident in 4.9%; renal complications in 11% (defined as an increase of at least 0.3 mg/dL or >1.5-2 fold in 48-h creatinine or diuresis <0.5 mL/kg); superficial infection of the surgical wound in 4.9%; and suture dehiscence in 2.4%. None of the patients needed repeat intervention for bleeding or for surgical or medical complication.
Univariate analysis revealed the following variables to be significantly associated with mortality in the immediate postoperative period: female sex (P=.002) and mild preoperative mitral insufficiency (P=.05). Interestingly, no statistically significant association with mortality was observed for surgical times, percentage of patients with Euroscore >10, or the presence of a patient-prosthesis mismatch (Table 2). A nonsignificant trend towards increased mortality was observed in cases of mismatch; this effect probably failed to reach statistical significance due to the limited number of cases.
In the multivariate analysis, including all factors associated in the literature with perioperative morbidity and mortality (coronary lesions, mean gradient, acute myocardial infarction [AMI]), the only predictors of mortality in the immediate postoperative period were female sex (OR, 2.60; 95% CI, 2.20-89; P=.004), prior mitral regurgitation (OR, 2.37; 95% CI, 1.44-80; P=.020), and prior coronary lesions (OR, 2.09; 95% CI, 1.261-51; P=.027) (Table 3).
Mortality During Long-term Follow-up
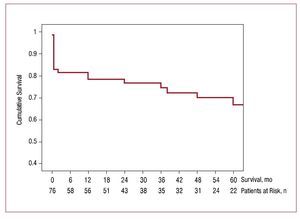
In addition to the 16 patients who died within 30 days of surgery, 12 patients (18.8%) died during a mean follow-up period of 42.59 (40.83) months (median [interquartile range], 30 [70-6.25] months). Two patients were lost to follow-up (97% follow-up in surviving patients). Of the 12 deaths occurring during follow-up, 5 were due to noncardiovascular causes (7.5%) and 7 were cardiovascular in origin (11.3%). The incidence of events (death) during follow-up was 2 per year. Figure 1 shows the Kaplan-Meier curve for total survival, indicating the number of patients at risk for each follow-up period.
Figure 1. Kaplan-Meier survival curve for all patients with severe aortic stenosis and ventricular dysfunction who underwent surgical valve replacement. The number of patients at risk are indicated for each time point during follow-up.
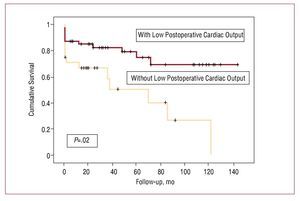
In the univariate analysis (Table 4), only postoperative complications (P=.03), particularly low postoperative cardiac output (P=.043) and pulmonary complications (P=.035), were associated with increased mortality during follow-up. In the multivariate analysis (Figure 2), only low postoperative cardiac output was associated with increased mortality during follow-up (OR, 4.40; 95% CI, 1.20-15.5; P=.02).
Figure 2. Kaplan-Meier survival curve according to the presence or absence of low cardiac output as a complication following surgical valve replacement in patients with severe aortic stenosis and ventricular dysfunction.
Improvement in Functional Class
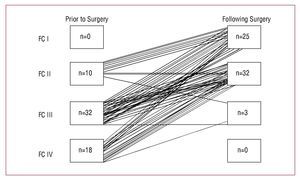
There was a symptomatic improvement in FC in most patients (93.4%) who survived surgical AoVR. Of the 60 patients for whom FC status was available prior to surgery and during long-term follow-up, 83.3% were in New York Heart Association FC IIIIV prior to surgery. After a mean follow-up of 42.59 (40.83) months (median [IQR], 30 [70-6.25] months), only 5% of patients remained in FC III and none in class IV (Figure 3).
Figure 3. New York Heart Association functional class (FC) before and after surgery. Improvement in functional class was observed during follow-up in 93% of patients.
Improvement in Ventricular Function
In addition to preoperative echocardiograms, postoperative echocardiograms were also available prior to discharge in 67 of 82 patients, and long-term echocardiographic follow-up was available in 33. Early improvement in ventricular function was observed in 70.5% of patients. In the univariate analysis, the following variables predicted absence of improvement: prior AMI (P=.04), coronary lesions without revascularization (P=.04), and reduced mean aortic gradient (P=.02). Only one case displayed delayed improvement.
DISCUSSION
In our study, we found that immediate postoperative mortality displayed a statistically significant association with female sex, prior mitral valve insufficiency, and concomitant coronary lesions. Likewise, in the multivariate analysis, the appearance of postoperative complications, specifically the development of low cardiac output, was significantly associated with long-term mortality. The absence of improvement in ventricular function was associated with prior AMI, the presence of untreated coronary lesions, and a reduced mean aortic gradient. These findings are consistent with previous reports. However, little information is available on surgical outcomes and long-term follow-up in large patient series. Many of the studies published to date included patients with AoS and regurgitation.12-14 Furthermore, many of the surgical series found in the literature are old and the surgical techniques, especially myocardial protection, have advanced since they were published.
In severe AoS, the left ventricle compensates for the chronic pressure overload through hypertrophy in an effort to normalize the wall stress. Initially, the ejection fraction (EF) and cardiac output are unaltered. When the wall stress exceeds the compensatory capacity, ventricular function begins to deteriorate. Thus, when ventricular dysfunction occurs as a result of this increase in afterload, as is the case in AoS, valve replacement will lead to increased EF and symptomatic improvement so long as there are no other causes of ventricular dysfunction (AMI, concommitant valve disease, etc).2
Effect of Valve Replacement on Mortality
Mortality in the Immediate Postoperative Period
In our study, we identified female sex as an independent factor associated with immediate postoperative mortality. The prognostic influence of sex in patients undergoing valve replacement for severe AoS has been subject to debate. Although it has been suggested to play an important role as an independent marker of risk during cardiac surgery,15 when the data are adjusted for body surface area, some studies suggest that the effect disappears, particularly in patients with AoS.16
Prior mild mitral insufficiency has been characterized less extensively in the literature, but mild preoperative mitral regurgitation has been associated with postoperative mortality in AoS.17 Our study confirms these findings in patients with severe AoS and severe ventricular dysfunction. We suggest that mitral regurgitation may be a marker for severe ventricular dysfunction, which is also associated with geometric abnormalities that may imply an additional, potentially irreversible, factor in the development of ventricular dysfunction. This would explain its association with worse prognosis.
We also identified significant coronary artery disease as a predictor of in-hospital mortality. This finding is in agreement with the results of Connolly et al,4 who identified concomitant coronary heart disease as the only predictor of in-hospital mortality in a large patient series. The increased mortality could be related to the failure to revascularize concomitant coronary lesions, the longer periods of ischemia and on-pump time required for valve replacement in combination with bypass surgery, or the presence of concomitant coronary lesions and prior AMI. Nevertheless, in the analysis of our patient series, the absence of revascularization, prolonged periods of ischemia, and history of AMI were not associated with increased mortality.
Notably, in the literature, patient-prosthesis mismatch tends currently to be identified as an independent predictor of short-term morbidity and mortality in patients undergoing AoVR for severe AoS, and this effect appears even more pronounced in cases of severe ventricular dysfunction.18,19 Although fewer data have been published, it even seems to have been identified as a predictor of long-term survival.20 In our study, we observed a trend towards increased mortality in cases of patient-prosthesis mismatch, but this did not reach statistical significance, probably due to the very small sample size and number of deaths observed.
Death During Follow-up
We only identified serious postoperative complications (low cardiac output and pulmonary complications) as a predictor of long-term mortality. Low postoperative cardiac output was identified as the only independent variable. In these patients, low cardiac output may be related to postsurgical appearance of dynamic subaortic obstruction, pump failure, vasoplegic syndrome, bleeding, or a combination of those possibilities. This complication could not be analyzed systematically, but repeat intervention as a result of bleeding was assessed, and no cases were found in our patients. On the other hand, subaortic dynamic obstruction is probably a less common complication during follow-up in patients with severe ventricular dysfunction, especially those with reduced EF and ventricular dilation.21 Another possibility is that patients with low cardiac output as a complication have a longer and less-favourable postoperative course (more than 1 month), leading to higher mortality in the first 6 months. However, as shown in Figure 1, this effect persists not only in the first few months but throughout follow-up.
Effect of Valve Replacement on Ventricular Function and Functional Class
Aortic valve replacement for treatment of AoS reduces ventricular afterload.22 This affects subsequent ventricular adaptation and remodeling, with regression of hypertrophy and reduction of ventricular mass.23 Consequently, an improved EF can be expected after AoVR in patients with diminished preoperative EF.17,24 If that improvement does not occur, it is likely that there is preexisting irreversible myocardial damage. Previous studies have demonstrated that reduced postoperative EF, prior AMI, and low postoperative aortic valve gradient are associated with reduced postoperative EF.25
The association between significant coronary artery disease and the absence of improvement in ventricular function following AoVR has been described previously13,26 and is explained both by the increased risk of perioperative AMI and by an increase in the incidence of irreversible myocardial damage or scarring prior to valve replacement.
A low mean aortic valve gradient prior to surgery is also associated with the absence of postoperative improvement in EF. This low gradient indicates a reduced capacity of the myocardium to generate a high pressure gradient across the stenotic valve, and in many cases reflects a myocardium with little capacity for recovery despite the improvement in afterload as a result of valve replacement.4 Some recent studies have focused on the subgroup of patients with severe AoS, ventricular dysfunction, and low transvalvular gradient.27,28 Although this defines a group of patients at increased risk and with worse prognosis (consistent with our study, in which this subgroup of patients had less improvement in EF), surgery is still recommended, given the poor results achieved in patients without surgery.28 In these patients, dobutamine stress echocardiography facilitates selection of patients with contractile reserve.27,28
Despite the high initial perioperative mortality, patients who survive surgery exhibit clear clinical improvement with greater medium-term and long-term survival. This is consistent with the results of other studies and indicates that ventricular dysfunction alone should not be a contraindication for surgery.29
Surgical Versus Percutaneous Aortic Valve Replacement
It is possible that new therapeutic techniques, such as percutaneous implantation of aortic valve prostheses, may be useful in patients with AoS and ventricular dysfunction, given the perioperative mortality seen in this group. However, large patient series and long-term follow-up have yet to be published, thus limiting the possibility of assessing percutaneous approaches as possible alternatives to surgery beyond their current indications.30,31
Limitations
This was a retrospective study. The small sample size (82 patients) and the limited number of deaths observed (16 in the immediate postoperative period and 12 during subsequent follow-up) limit the quality of the results that could be obtained (leading to ORs with large 95% CIs and reducing the statistical power for the identification of predictors of events). Echocardiographic follow-up could not be completed in 30 patients (47.6% of surviving patients) for a variety of reasons, including lack of acces to echocardiographic data and patients from outside of Spain. It was not possible to analyze in detail the principal causes of low cardiac output in the majority of the cases analyzed, thus reducing the availability of information that could help to explain the relationship between this complication and mortality.
CONCLUSIONS
Despite considerable mortality during the immediate postoperative period in patients with AoS and severe left ventricular dysfunction, good long-term survival is observed, along with improved ventricular function and FC. There is a statistically significant association between mortality during the immediate postoperative period and female sex, prior mitral insufficiency, and concomitant coronary lesions. The occurrence of postoperative complications, specifically the development of low cardiac output, is significantly associated with long-term mortality.
ABBREVIATIONS
AMI: acute myocardial infarction
AoS: aortic stenosis
AoVR: aortic valve replacement
EF: ejection fraction
FC: functional class
Correspondence: Dr. J.J. Gómez-Doblas.
Área del Corazón. Hospital Universitario Virgen de la Victoria. Campus Teatinos, s/n. 29010 Málaga. España.
E-mail: jjgomezdoblas@secardiologia.es
Received January 4, 2009.
Accepted for publication October 7, 2009.