Keywords
INTRODUCTION
Prosthetic valve endocarditis (PVE) is a serious, although uncommon, complication in patients who have undergone valve replacement.1,2 Despite recent progress in its diagnosis and treatment, PVE presents high morbidity and mortality.3-5 In fact, in PVE, mortality ranges between 25% and 59%, although there has been unquestionable progress in the prophylaxis, diagnosis and treatment of this disease.6-9
The purpose of this work was to analyze changes in the epidemiological factors, etiology, and clinical characteristics of patients with PVE, and to study their treatment and prognosis.
METHODS
The study was conducted in the Hospital Universitario Marqués de Valdecilla of Santander (Cantabria, Spain), a tertiary-care hospital with an approximate capacity of 1200 beds. During the study period, this was the referral hospital for cardiovascular surgery in this Autonomous Region (Cantabria, approx. 520 000 inhabitants) and for other nearby regions (Castilla and León, Galicia, Asturias, the Basque Country, and La Rioja). It conducts approximately 1000 cardiovascular surgical procedures per year, of which 500 involve cardiac surgery.
This retrospective cohort study was conducted in the Hospital Universitario Marqués de Valdecilla between 1986 and 2005 and included all patients over 14 years of age diagnosed with PVE. Cases were identified retrospectively by analyzing the computerized registries of Cardiovascular Surgery Infectious Diseases and Admissions and Clinical Documentation Service. Prosthetic valve endocarditis was defined according to modified Duke criteria10 with additional modifications specific to diagnosing PVE, made by our working group.11 Only patients with a definitive diagnosis of PVE who had previously undergone valve replacement surgery in our hospital were included. Patients who had any prior episode of PVE were considered as new cases. The classic criterion of 60 days from surgery to PVE symptom onset was employed to differentiate early onset from late onset.12 All patients included in the study underwent follow-up from the time PVE was diagnosed until hospital discharge.
With the aim of analyzing the epidemiological and clinical differences, the study was divided into 2 periods: period 1 (P1), from January 1986 to December 1995, and period 2 (P2) from January 1996 to December 2005. This provided 2 periods of equal length, the second of which included our hospital's introduction of transesophageal echocardiography was introduced in our hospital to diagnose PVE (from 1995 onwards). P1 was regarded as the reference period in the statistical analysis.
Definitions
The medical histories of the patients were retrospectively examined to analyze the following factors:
- Epidemiological data: age, sex, place of origin (Cantabria or other region); previous endocarditis, previous valve surgery (type of surgery, duration and type, size and position of valve); underlying diseases, (COPD, kidney failure, diabetes mellitus, liver disease, previous stroke and neoplasms); comorbidity, defined by the presence of two or more of the following factors: diabetes mellitus, age >75 years, human immunodeficiency virus (HIV) infection, cancer, and immunodeficiency.8
- Clinical findings: fever, new murmur or changes in the characteristics of known murmur, heart failure, and neurological alterations, as well as complicated PVE, bad outcome, uncontrolled infection, septic shock, and in-hospital mortality. "Complicated" PVE followed criteria developed by Calderwood et al13 (new murmur, heart failure, fever for more than 10 days, or cardiac conduction abnormalities). "Bad outcome" was applied in cases of relapse, valve surgery related to sequelae of the infection, or death with indications of unresolved infection or prosthesis dysfunction.13 "Uncontrolled infection" was defined by prolonged fever, persistent bacteremia or both following 1 week of appropriate antimicrobial treatment.14 "Septic shock" was defined as prolonged hypotension (systolic blood pressure ≤90 mm Hg), not due to cardiogenic shock, together with at least one of the following perfusion abnormalities: oliguria, acute mental alterations, or lactic acidosis.15 "In-hospital mortality" was defined as death during initial hospitalization for infectious endocarditis.14
- Microbiological data: blood cultures, date of acquisition, number of samples extracted and positive samples, and the results of serological studies and cultures of the infected valve, when available.
- Echocardiography: the presence of vegetations, prosthetic dysfunction, prosthetic dehiscence, and myocardial abscesses on transthoracic or transesophageal echocardiograms..16,17
Statistical Analysis
The recorded data were entered into a database created with SPSS 15.0. The Student t test was used to compare between-group means for quantitative variables. Qualitative variables were compared using the c2 test or Fisher's exact test (when the expected frequencies were <5). The size of the effect was determined by calculating the relative risk (RR) with 95% confidence interval (CI), taking P1 as the reference. Differences were accepted as significant when a errors <.05.
RESULTS
A total of 133 episodes of PVE occurred in 122 patients; 112 patients had a single episode, 9 had 2 episodes, and 1 patient had 3 episodes. During the 20-year study period, there were 6079 valve replacement procedures, with an approximate cumulative incidence of PVE of 2.2% in our hospital. Of the 133 episodes studied, 73 (54.9%) cases of PVE were diagnosed during P1 (1986-1995) and 60 (45.1%) during P2 (1996-2005). In total, 65% of the patients were more than 65 years of age at the time of diagnosis.
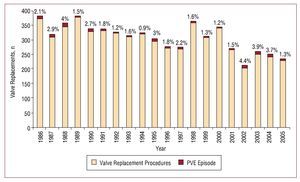
During P1, 73 episodes of PVE occurred out of 3330 procedures (incidence, 2.19%). During P2, 60 episodes of PVE occurred out of 2749 valve replacement procedures (incidence, 2.18%). Figure shows how the number of cases of PVE evolved over time in relation to the total number of valve replacement procedures.
Figure 1. Bar chart indicating in each column the percentage of prosthetic valve endocarditis episodes in relation to valve replacement procedures conducted at the Marqués de Valdecilla University Hospital during the 2 study periods. PVE indicates prosthetic valve endocarditis.
Analysis showed that the population was aging, with a mean (standard deviation) of 52.6 (16.6) years during P1 and 66.2 (11.5) years during P2 (P=.0001). A significant decrease in the number of men was observed (P1, 75 [3%]; P2, 35 [58.3%]; RR=0.8; 95% CI, 0.6-1; P=.04). Table 1 shows the epidemiological characteristics of the study population.
Regarding valvular heart disease prior to surgery, a decrease was observed in the incidence of rheumatic heart disease and an increase in degenerative valvular heart disease (Table 1). Similarly, there was a decrease in the percentage of bioprosthetic valves implanted: P2, 23 (39.7%), versus P1, 44 (62%) (RR=0.6; 95% CI, 0.4-0.9; P=.012).
In relation to risk factors, the only significant finding was the increase in the incidence of diabetes mellitus (P2 vs P1, 15% vs 1%; RR=10.9; 95% CI, 1.4-84; P=.003), but no significant differences were observed between the 2 periods regarding any other factors analyzed (Table 1).
Late onset PVE occurred in 109 cases (82%) and early onset PVE in 24 (18%). Of the 109 late onset cases, 22 (20.2%) occurred between 3 and 12 months after valve replacement, 20 (18.3%) occurred between 12 and 36 months, and 67 (61.5%) occurred 36 months after the procedure. No significant differences were observed in the incidence of early onset PVE and late onset PVE in the 2 study periods analyzed (early onset PVE, P2 vs P1, 18.3% vs 17.8%; RR=1; 95% CI, 0.5-2.1; P=.94).
The most relevant clinical data at the time of diagnosis were as follows: fever, present in 80% of patients and more frequent in late onset PVE (84%) than in early onset PVE (71%); heart failure, present in 65% of patients and, in contrast to fever more prevalent in early onset PVE (75%) than in late onset PVE (64%); new murmurs or qualitative changes in existing murmurs, appearing in 64% of patients and more frequent in late onset PVE (66%) than in early onset PVE (54%). Other clinical signs and symptoms did not vary significantly during the study period.
Blood cultures were performed for all episodes. In 24 patients (18%) blood cultures were negative, and no significant differences were observed between the 2 study periods (P2 vs P1, 16.4% vs 12%; RR=1.2; 95% CI, 0.5-2.7; P=.63). Table 2 shows the microorganisms isolated in the 82% of cases with positive blood cultures: coagulase-negative Staphylococcus in 36 cases (33%; 31 cases of S epidermidis); viridans-group Streptococcus, 25 cases (22.9%), Staphylococcus aureus, 17 cases (15.6%; 12 cases of methicillin-sensitive S aureus [MSSA] and 5 cases of methicillin-resistant S aureus [MRSA]); and Enterococcus, 9 cases (8.3%); other bacteria, 17 cases (15.6%), and Candida spp., 4 cases (3.6%). Analysis of the microbiological findings showed only one significant difference between the 2 study periods (1986-1995 and 1996-2005), a decrease in viridans-group Streptococcus infections (P2 vs P1, 12.5% vs 31.1%; RR=0.4; 95% CI, 0.2-0.9; P=.02) (Table 2).
In total, 49.2% of the echocardiograms were transthoracic and 50.8% were transesophageal (24% of the echocardiograms were transesophageal during P1 and 84% during P2; RR=3.5; 95% CI, 2.2-5.5; P<.0001). Vegetations were present in 84 patients (63.1%); intrinsic prosthesis dysfunction in 95 (71.4%); valve dehiscence in 13 (9.7%); and myocardial invasion in 39 patients (29.3%). There were no significant differences in echocardiographic findings between P1 and P2.
A total of 107 patients (80.5%) received surgical treatment, with more surgeries during P1 (90.4% [66/73] vs 68.3% [41/60] in P2; RR=0.8; 95% CI, 0.6-0.9; P=.001).
Complicated bacterial endocarditis was present in 96 (72.2%) patients, uncontrolled infection in 19 (14.3%), and bad outcomes in 28 (21.1%). Hospital mortality occurred in 39 patients (29.3%) and was due to early onset PVE in 41.7% (10/24 patients) and late onset PVE in 26.6% (29/109 patients); no significant differences were observed between the 2 periods (30% during P2 and 28.8% during P1; RR=1; 95% CI, 0.6-1.8; P=.88).
DISCUSSION
The present study was conducted over a long period, due in part to the particular characteristics of PVE, which has low incidence. This approach offered the opportunity to compare 2 defined periods and analyze changes in the epidemiology of the infection and the impact of changes in medical practice on the microbiology and prognosis of PVE. A review of the literature revealed that the series presented here is the widest using definitive criteria for PVE in a single hospital. Although various multicenter studies have recruited more patients,18,19 our series has two signature advantages with respect to the homogeneity of both data gathering and clinical practices: a single person conducted the data collection and the composition of the medical and surgical teams effectively remained the same throughout the 20-year study period. In this regard, the work of Pablo Rivas et al20 is worth mentioning due to its similarity to the present study, although their data collection period was longer (1970-2003). In our study, the mean age was 59 years at the time of diagnosis and population aged significantly between P1 and P2: 52.6 years vs 66.2 years , respectively. Furthermore, there were significantly more patients >65 years during P2. Following our review of the literature on PVE studies, the gradual aging of the population was confirmed (Table 3), and it is noteworthy that after 1990 the mean age of PVE patients increased by around 9 years.7-9,13,14,21-33
Analysis of early onset PVE (20%) and late onset PVE (80%) found no statistically significant differences between the 2 study periods (1986-1995 and 1996-2005) and a similar distribution in the percentage of early onset PVE and late onset PVE. This contrasts with the findings of two o other studies,20,34 which report a gradual decrease of early onset PVE and a slight increase in late onset PVE, although both of these studies were begun in the 1970s, which may explain the differences.
Historically, PVE has predominantly been a disease of patients with underlying rheumatic heart disease5,36 and community-acquired bacteremia, and the most frequent etiology was Streptococcus infection, involving 60% to 80% of all patients. On the other hand, the prevalence of rheumatic heart disease has decreased in recent years,37,38 whereas the prevalence of chronically ill patients undergoing invasive processes has increased.39 In the present study, analysis of the 2 periods showed a gradual decrease of rheumatic heart disease and an increase in degenerative valvular heart disease.
During the last 20 years, various authors have suggested that changes in the epidemiological characteristics of the population may have played a decisive role in the clinical characteristics and prognosis of this disease.37,40,41 In particular, the steady increase in the use of invasive procedures (surgical techniques, central venous catheters, hemodialysis), with the consequent increase in hospital-acquired infections, has been implicated as a factor that can increase the rate of endocarditis.42 On the other hand, progress in echocardiography and the use of validated diagnostic criteria have led to improvements in the diagnosis of this disease.10,43
Although descriptive studies on the epidemiology of endocarditis were conducted in the 1980s and 1990s,44-46 few studies have works focused on the association between changes in epidemiological characteristics and prognosis. Cabell et al,41 of Duke University, attempted to identify changes in the epidemiological and microbiological characteristics of patients with endocarditis, with the further aim of determining their effect on survival. This study, like all previous ones, had the methodological limitation of jointly analyzing episodes of native valve endocarditis and PVE. Nevertheless, this was an extensive series with 329 patients, 30% of whom had PVE. Over the 7-year study period, it became clear that there was an increase in the number of immunodeficient patients and hemodialysis procedures, which implied more infections caused by Staphylococcus aureus in contrast to a decrease in endocarditis caused by viridans-group Streptococci. Similarly, endocarditis due to Staphylococcus aureus was associated with greater patient mortality. In the present study, we also found a greater number of patients with diabetes mellitus, immunodeficiency, and kidney failure during P2.
Regarding the microbiology of prosthetic valve infections, we would like to emphasize our agreement with the experience of most authors who report S epidermidis and S aureus as the cause of most episodes of both early onset PVE47 and late onset PVE,4 and viridans-group Streptococci as the cause of a sizable number of late onset cases. However, we did not observe the preponderance reported in more recent studies of Staphylococcus aureus.14,41,48 In our series, the increase in Enterococcus infections in P2 was striking, as well as the decrease in viridans-group Streptococcus infections. In particular, the number of S aureus infections remained similar during the 2 periods analyzed (14.7% vs 12.3%), in contrast to the work of Rivas et al,20 which reported an increase in PVE caused by Enterococcus and S aureus in the case of late onset PVE.
The introduction of transesophageal echocardiography in the 1990s revolutionized the echocardiographic diagnosis of PVE due to its greater diagnostic sensitivity (76%-100%) and its specificity (94%) in assessing the perivalvular extent of infection49; thus, it is very helpful in diagnosing and monitoring the disease, and in deciding which patients should undergo reintervention.16,49,50 Our study reflects this change in the echocardiographic approach to PVE, with transesophageal echocardiography being used in 84% of cases during P2. This improvement in the diagnosis of PVE could explain the lower number of surgical interventions during P2, since this may have led to improved selection of those patients requiring surgery.
In total, 29% (38 patients) died in hospital due to complications of PVE. The in-hospital mortality rate was 41.7% in early onset PVE and 26.6% in late onset PVE; no statistically significant differences were found between 1986-1995 and 1996-2005. Studies on mortality in patients with PVE are scarce. Furthermore, some of the few available studies jointly analyzed mortality in native valve endocarditis and PVE or, on the other hand, mortality specifically associated with PVE episodes caused by certain microorganisms, particularly S aureus.48 Our figures are similar to those of other published studies, which indicates the severity of this disease and the possible influence of the changes in the epidemiological and microbiological characteristics of the patients. Mortality is particularly high in early onset PVE,51,52 with rates ranging between 40% and 75%, and is associated with invasive microorganisms that cause abscesses and destruction of the valvular ring and, thus, a greater number of cardiac complications.53
This paper has certain limitations that should be mentioned. The study was retrospective and conducted in a single hospital, which in a certain sense limits the validity of the results, although the cases of PVE had been prospectively recorded in the computerized registries of the Cardiovascular Surgery and Infectious Disease services. Furthermore, the incidence of PVE was not calculated in a strict sense. Although all the new cases of PVE diagnosed during the study period were included in the numerator, the 6079 operated patients were not followed up, and it is possible that some of them contracted PVE and were not referred to our hospital again, although this is not customary clinical practice. On the other hand, the study was conducted only on patients with a definitive diagnosis of PVE and has the advantage of analyzing longitudinally the evolution of this disease ever a long period and indicates the changes in the characteristics of the disease.
CONCLUSIONS
This work, conducted over a 20-year period of treating patients with PVE, reports a gradually aging population with PVE, changes in underlying valvular heart disease and a gradual decrease in the etiology of Streptococcus infection, but with no decrease in mortality.
ABBREVIATIONS
CI: confidence interval
PVE: prosthetic valve endocarditis
RR: relative risk
Correspondence: Dr. M.C. Fariñas.
Unidad de Enfermedades Infecciosas. Servicio de Medicina Interna. Hospital Universitario Marqués de Valdecilla.
Avda. Valdecilla, s/n. 39008 Santander. Cantabria. España.
E-mail: farinasc@unican.es; mirfac@humv.es
Received March 20, 2009.
Accepted for publication October 7, 2009.