Keywords
INTRODUCTION
The natural course of severe aortic stenosis (SAS) is well known1; aortic valve replacement (AVR) is the only reliable treatment. Severe aortic stenosis is the most common reason for valve surgery in Spain.2
The associated perioperative mortality associated with valve replacement varies.2-4 Many factors have been associated with increased perioperative morbidity/mortality in patients who undergo AVR, the most significant of which include previous revascularization surgery, the need for emergency surgery, advanced age, serious left ventricular dysfunction (ejection faction [EF] <40%), kidney failure, advanced heart failure (functional grade III/ IV), and a small body surface area (BSA).5,6 Although sex is generally recognized as a negative prognostic factor in heart surgery,7 there is little information regarding its influence in patients who undergo valve surgery for SAS.
The aim of the present work was to analyze whether female sex is an independent predictive factor of perioperative morbidity/mortality.
METHODS
Study Population
This retrospective study examined the data of a cohort of patients who underwent AVR for SAS between February 1996 and April 2007. All exclusively received a prosthesis (biological or mechanical) in the aortic position. The following patients were excluded from the study: a) those who had undergone AVR for predominant aortic regurgitation or coronary disease with a concomitant, non-severe aortic valve lesion; b) those who had undergone valve replacement in the context of type A aortic dissection with valve involvement or other involvement of the ascending aorta; c) those who had undergone replacement or repair of another heart valve; d) those with mitral or tricuspid valve disease of rheumatic or endocarditic origin, or with prolapse of any origin; and e) those with systolic anterior motion caused by left intraventricular dynamic obstruction.
The demographic, epidemiological, clinical, electrocardiographic, and echocardiographic data of all patients were collected. Ventricular hypertrophy was considered present when the interventricular septum was >12 mm across; this was classified qualitatively. Also recorded were the results of any coronary angiography performed, the type and size of the implanted prosthesis, morbidity/mortality during the perioperative period (defined as the time between surgery and 30 days post surgery), and the need for reintervention.
The variables analyzed (cardiovascular risk factors, patient antecedents, and postoperative complications) were defined in accordance with the standards of the American College of Cardiology/ American Heart Association.8
Doppler Echocardiography
Patients underwent echocardiographic examination prior to surgery using Acuson Sequoia (Siemens Co.), Acuson Aspen (Siemens Inc.), or VingMed 750 (GE) apparatuses. Standard examination included M-mode, 2-dimensional (2D), color and spectral Doppler recordings in the normal planes (including the long and short parasternal axes, and the apical 3-, 4-, and 5-chamber planes). Adhering to the norms of the American Society of Echocardiography9 (ASE), analyses were made of the following: aortic valve variables (maximum and mean gradient and valve area estimated using continuity equations, and the presence of aortic regurgitation), mitral valve variables (morphology and function), and tricuspid variables (the presence and degree of left ventricular hypertrophy, systolic function, and the pulmonary systolic pressure [when possible]).
The severity of mitral regurgitation was estimated semi-quantitatively taking into account the regurgitation flow area as determined by color Doppler, pulsed Doppler tracing, and the flow in the pulmonary veins, as described in the norms of the ASE.10
Statistical Analysis
Continuous variables were expressed as means (standard deviations). Qualitative variables were expressed as percentages. The c2 was used to analyze differences between qualitative variables, and the Student t test to analyze those between continuous variables. A P value less than .05 was considered significant. Multivariate analysis (multiple logistic regression) was performed to identify variables that were independent predictors of perioperative mortality. This was undertaken in a stepwise fashion to more clearly highlight their association. All variables that were significant in univariate analysis were included in this analysis, as were those recognized as predictors in the literature. Corresponding odds ratios (OR) and 95% confidence intervals (95% CI) were calculated.
RESULTS
Population
The study population was made up of 577 patients. All had undergone AVR during the study period; all met the inclusion requirements. The mean age was 68.3 (9.2) years; 44% of the patients were women. The incidence of cardiovascular risk factors was as follows: 59.1% of patients had high blood pressure, 25.3% had a background of smoking or were active smokers, 28.4% had diabetes mellitus, and 21.8% had dyslipidemia. Some 73.1% suffered dyspnea, 42.8% suffered angina, 12.7% suffered exercise-induced syncope, and 56.9% had heart failure.
Baseline Characteristics
Table 1 shows the baseline characteristics of the patients. Before surgery, 68.5% of patients showed sinus rhythm; 5.4% had suffered a prior acute myocardial infarction (AMI). Coronary angiography was performed on 92.6% of patients before surgery, among whom 26.3% showed significant coronary lesions. The baseline echocardiographic characteristics of the population included a maximum gradient of 79.2 (23.1) mm Hg and a mean gradient of 55 (17.8) mm Hg. Some 34.2% of patients showed non-severe mitral regurgitation, 19.2% suffered tricuspid regurgitation, and 85.8% had some degree of ventricular hypertrophy. The overall mean EF was 60.3% (12.3%) (Table 1).
The study population included 254 women and 323 men. The men had a lower mean age than the women (66.8 [9.8] years compared to 70.3 [7.9] years; P<.001), and a greater body surface area (BSA) (1.83 [0.16] m2 compared to 1.68 [0.15] m2; P<.001). Fewer men suffered high blood pressure (49% compared to 73% of the women; P<.001), diabetes mellitus (24.5% compared to 33.5%; P=.001), heart failure (52.9% compared to 62.1%; P=.027) or ventricular hypertrophy (83.1% compared to 89.1%; P<.001). However, a greater proportion of men showed significant coronary disease (31.8% compared to 19.1%; P<.001) and severe ventricular dysfunction (17.4% compared to 7.9%; P<.001) (Table 1).
Surgical Results
A total of 297 patients (51.5%) received a biological prosthesis (18.8% with no support ring). Some 26.3% of the entire patient sample showed significant coronary lesions; concomitant aortocoronary bypass was performed in 79.9% of those in whom it was indicated; this was not performed in the remaining 20.1% because of technical difficulties (diffuse lesions, small vessels, or total occlusion and vessel inviability); no differences were seen between the men and women in this respect (19.3% among the women compared to 23.5% in men; P=.577).
Total Mortality
Univariate analysis showed age to be significantly related to mortality (the mean age of those who died was 72.1 [7.6] years compared to 67.9 [9.2] years for those who did not; P=.044), as was prior AMI (14.6% of those who died had suffered a prior AMI compared to 5.5% of those who survived; P=.034), a reduced EF (56.1% [13%] compared to 60.7% [12%]; P=.010), non-severe mitral regurgitation (54.1% compared to 32.5%; P=.008), the need to perform an associated coronary procedure (31.6 compared to 18.8%; P=.023), and female sex (13% in women compared to 7.4% in men; P=.019). Other factors related to mortality were tricuspid regurgitation, high systolic pulmonary pressure, atrial dilation, and the implantation of biological prosthetic valves (Table 2). The BSA was not related to mortality (1.76 [0.17] m2 in those who died compared to 1.75 [0.19] m2 in those who did not; P=.730), nor was the need for postoperative reintervention (P=.149). Only 2 patients underwent emergency AVR surgery; both died during the postoperative period (Table 2).
In multivariate analysis (which included the classic factors believed associated with perioperative morbidity/mortality) the variables female sex (OR=2.22; 95% CI, 1.01-4.90; P=.048) and reduced EF (OR=2.81; 95% CI, 1.05-7.48; P=.039) were initially found to be independently related to increased mortality (Tables 3 and 4). However, when BSA was included in the analysis, the significance of female sex disappeared (OR=2.40; 95% CI, 0.79-7.26; P=.119) and non-severe mitral regurgitation showed a trend towards being a marker of poor prognosis (OR=2.09; 95% CI, 0.99-4.41;P=.053) (Table 5).
Morbidity With Respect to Sex
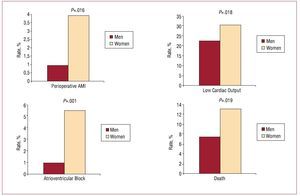
Compared to the men, the women patients were at no greater surgical risk according to the EuroSCORE and Parsonnet risk stratification scales (P=.313 and P=.308 respectively). However, in general, complications were more common in the women; they showed more cases of prior AMI (3.9% compared to 0.9%; P=.016), more often suffered postoperative atrioventricular block (5.5% compared to 0.9%; P=.001) and more often showed low cardiac output (30.3% compared to 22.3%; P=.016) (Figure).
Figure. Perioperative complications with respect to sex. AMI indicates acute myocardial infarction.
When taking BSA into account in multivariate analysis of postoperative complications with respect to sex, only atrioventricular block was found to be more common among the women than the men (OR=9.608; 95% CI, 1.57-58.62; P=.014); the differences shown in cardiac output in univariate analysis disappeared (OR=0.98; 95% CI 0.52-1.84; P=.953) (Table 6).
DISCUSSION
Total perioperative mortality in the studied cohort was 9.9%, but was greater among the women (13%) than the men (7.4%). The same trend was seen with respect to perioperative complications. Classically, perioperative morbidity/mortality has been associated with prior coronary revascularization surgery, the need for emergency surgery, advanced age, severe left ventricular dysfunction, kidney failure, advanced heart failure (functional grade IIIIV/IV), and non-severe mitral regurgitation,6,7,11,12 although only severe left ventricular dysfunction and the need for emergency surgery are well accepted markers of mortality. In recent years, several studies have reported female sex to be a risk factor of perioperative morbidity/mortality in AVR surgery. Indeed, female sex has begun to become considered a "cause" of greater perioperative mortality in heart surgery. The reasons why female sex should be a risk factor are not clear,13 although the clear pathophysiological differences in SAS between men and women14 may be one of the main determining factors. Evidence of the greater perioperative morbidity/mortality of women who undergo heart surgery is provided by several registries. These mainly concern coronary surgery15,16 but also represent valve replacement surgery.8 In these registries, female sex behaves as an independent predictor of perioperative mortality. However, not all studies report exactly the same findings, and some insinuate that the increase in morbidity/mortality among women is not just a question of sex.17 Unfortunately, there have been few studies that have looked into this in depth, and those that have been undertaken have commonly left aside the difference in BSA between men and women. It has been reported that patients with a smaller BSA or who are less tall suffer greater perioperative mortality.6,7 Along with factors already thought related to increased perioperative morbidity/mortality (such as a reduced EF, non-severe mitral regurgitation or coronary lesions), female sex initially seemed to be an independent risk factor among the present patients, as well as a risk factor for atrioventricular block, a lower cardiac output, and a greater incidence of perioperative AMI. However, in stepwise regression analysis taking into account BSA, this negative prognostic influence of female sex disappeared (even though the women showed more perioperative morbidity/mortality). The BSA of the present women was significantly smaller than that of the men, but this was not related to mortality in either univariate analysis, univariate analysis stratified by sex, or multivariate analysis. This suggests that neither BSA nor sex alone are associated with greater morbidity/mortality, but rather with other factors that do show such an association. One that should be borne in mind is the different pathophysiological response of men and women to the pressure overload caused by aortic stenosis (it is reported that for equal transvalvular gradients women show a tendency towards greater ventricular hypertrophy).14 This conditions complications in the perioperative period.18-20 In the present work, although it was not directly related to mortality, ventricular hypertrophy (and probably low perioperative cardiac output) was more prevalent in the women patients, which might help increase morbidity in this sex.
In addition to these factors, one should bear in mind the different management required by patients with a smaller BSA. In the present work the women patients showed a greater prevalence of atrioventricular block during the perioperative period. This was probably related to their greater age, their greater calcification of the atrioventricular junction, their smaller aortic roots, the greater difficulty of implanting the prosthesis in women, and the greater damage caused to the interventricular septum. Providing adequate myocardial protection can also be difficult in women since their ventricles are smaller and tend to be more hypertrophied in SAS,21,22 favoring the appearance of a perioperative AMI.
Thus, although women who undergo AVR show greater perioperative morbidity/mortality, sex per se is not an independent prognostic factor of this, but a quality that encompasses different factors, for which specific and specialized treatment may be required.
Limitations
This is a retrospective study; the results do not, therefore, allow the factors that lead to the increase in morbidity/mortality in women to be identified.
CONCLUSIONS
Perioperative mortality in women with SAS who undergo AVR is high. Even so, sex per se is not an independent predictor of mortality when potential confounding factors, such as BSA, are taken into account.
SEE ARTICLESON PAGES 7-9
ABBREVIATIONS
AMI: acute myocardial infarction
AVR: aortic valve replacement
BSA: body surface area
SAS: severe aortic stenosis
Correspondence: Dr. J. Caballero-Borrego.
Parque Doña Sofía, bloque 4, portal 2, 6.o G. 29640 Fuengirola. Málaga. España.
E-mail: jcabbor1@hotmail.com
Received April 18, 2008.
Accepted for publication August 6, 2008.