Percutaneous closure of left atrial appendage (PCLAA) has been shown to be a safe alternative to long-term oral anticoagulation (OAC), mainly in patients with nonvalvular atrial fibrillation, high embolic risk, and contraindication for OAC. Data are now available from large series with long-term follow-up. Cases that may extend knowledge of the appearance of possible events in long-term follow-up are of interest to prevent complications.
We present 2 cases of late device-associated thrombosis (LDAT), detected at 3 and 4 years after implantation. Both patients received the Amplatzer Cardiac Plug (ACP) (St Jude Medical/Abbott; Minneapolis, Minnesota, United States). Both patients were asymptomatic and a thrombus adherent to the central pivot of the device was detected in routine transesophageal echocardiography (TEE).
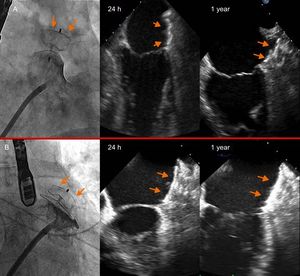
The first patient was a 61-year-old man with nonvalvular atrial fibrillation, gastroduodenal ulcer, and colon polyps in treatment with acenocoumarol. PCLAA was indicated for several episodes of gastrointestinal bleeding even though anticoagulation levels were within therapeutic range. He had a CHA2DS2-VASc score of 1 and a HAS-BLED score of 2. An ACP device no. 22 was implanted, with satisfactory angiographic and echocardiographic outcomes (Figure 1A). Indefinite treatment with aspirin 100mg and clopidogrel 75mg for 3 months was prescribed. TEE was performed according to the protocol of our center at 24hours, 45 days, 6 months, and 1 year (Figure 1A). Three years after implantation, echocardiography detected LDAT that was not present in the previous follow-up visits (Figure 2A, arrows).
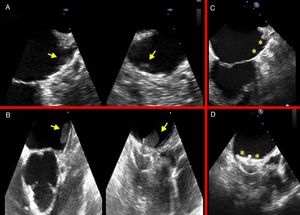
Thrombus adhered to the central screw of the Amplatzer Cardiac Plug device (arrows) in echocardiograms performed at 3 and 4 years in patient 1 (A) and patient 2 (B). Echocardiogram after 30 days of anticoagulation; a thrombus adherent to the device can no longer be detected (C and D, the asterisks indicate the smooth surface of the device).
The second patient was a 79-year-old man with colon polyps who was admitted for spontaneous subarachnoid hemorrhage (without antithrombotic treatment) and nonvalvular atrial fibrillation. PCLAA was indicated. He had a CHA2DS2-VASc score of 3 and a HAS-BLED score of 3. An ACP device no. 26 was implanted, with satisfactory angiographic and echocardiographic outcomes (Figure 1B). He was prescribed the same treatment as the previous patient. However, both antiplatelet agents had to be suspended after 3 months due to lower gastrointestinal bleeding, and subsequently, he received no such agents. At 4 years, in the follow-up TEE recorded according to our protocol, LDAT was observed, which was not present in the previous follow-up visits (Figure 2B, arrows).
Anticoagulant treatment with enoxaparin (1mg/kg every 12hours) was administered for 30 days in both patients and in both the thrombus disappeared from the device surface (Figure 2C and D, asterisks). Subsequently, the first patient was prescribed triflusal 300mg indefinitely while the second was prescribed aspirin 100mg. There was no recurrence of thrombosis.
Follow-up with TEE of patients after device implantation is not clearly defined in clinical practice guidelines. In the PROTECT AF clinical trial, TEE follow-up was performed at 45 days, 6 months, and 1 year after implantation.1 In a more recent recommendation made by expert consensus, the same schedule is maintained, and subsequent TEE is advised if embolic events occur.2
Thrombosis associated with the appendage-closure device is uncommon, with a mean incidence of 3.9% with all devices. This incidence is around 4.8% with ACP and is somewhat lower with the Amulet and Watchman devices (2.0%)3; the vast majority of cases described in the literature were diagnosed early (median, 1.5 months after implantation) because of the timing of follow-up TEE.3
In the PROTECT AF study, the overall incidence of thrombosis (5.7%) was higher at 6 and 12 months than at 45 days. This is in line with the results of our experience in the sense that a single follow-up is not reliable for determining the incidence of device thrombosis, as more thrombi are detected at 3 to 6 months than at 45 days.4 However, late thrombosis refers to those events that occur after the first 6 months and very late events to those occurring after the first year. The table in the supplementary material contains a literature review of late and very late thrombosis.
Although thrombosis associated with PCLAA devices is asymptomatic, the clinical repercussion is not clear and long-term follow-up with TEE is appropriate, particularly with the ACP, given the thrombogenicity of the central screw and also because of the good response to treatment. Design improvements in the new devices may, however, make LDAT events less frequent.