Keywords
INTRODUCTION
It has been 10 years since the implantation of endovascular prostheses (stents) in the thoracic aorta began. Although their exact role remains unclear, they are an efficient, safe alternative, above all in patients at high surgical risk. Endovascular treatment is less invasive than traditional surgery, reduces hospitalization, and is associated with lower incidence of paraplegia and mortality.1
The technique's success is related to the accurate knowledge of aortic anatomy and the choice of stent size. This, in turn, depends on knowledge of fixation point diameters. Intravascular ultrasound (IVUS) allows us to study the anatomy and take measurements. The advantage of IVUS over other imaging techniques is that it can be used in the cardiac catheterization laboratory and during implantation.2 However, IVUS measurements in the thoracic aorta have not been sufficiently validated.
The objective of the present study it to compare IVUS measurements of the aortic lumen with angiographic measurements by computerized tomography (CT) or magnetic resonance (MR), which are considered the gold standards.
METHODS
Between March 2005 and June 2008, we performed IVUS studies on 13 patients (5 women; mean age, 48 [23-73] years) with thoracic aorta diseases: coarctation (7), aneurysm (3), and dissection (3).
We used non-steerable tip Ultra ICE® 9 Fr 9 MHz catheters (EP Technologies, Boston Scientific Corp., California, USA) connected to an iLab or Galaxy console (Boston Scientific Corp.). A 0.035 cm teflon guidewire was introduced using femoral artery access. Over this, a Convoy® delivery catheter, 11 Fr, 60 cm, 55° distally-curved sheath with radio-opaque tip (Boston Scientific Corp.) was inserted and moved forward up to the ascending aorta.
The ultrasound catheter was advanced along the sheath in a proximal direction until the aortic valve could be seen. Then, it was manually withdrawn until the distal end was 2 cm from the tip of the sheath. In this position, we slowly withdrew the IVUS sheath exploring the branch terminals, visceral branches, and pathologic segments, as far as the iliofemoral territory. During exploration, we optimized the catheter position in an attempt to locate it in the central line of the vessel, reorientating it with tiny gyrating movements of the sheath.
The IVUS images were stored digitally for subsequent analysis by an experienced cardiologist. Selected aorta segment diameters were measured semi-automatically with a validated QCU program (CMS 6.1, Medis, Leiden, The Netherlands).3
Angiographic studies were conducted by 16-detector CT (Siemens Sensation 16®, Erlangen, Germany) and/or 1.5T MR (Siemens Magnetom Avanto®, Erlangen, Germany). The CT angiography used iodinated contrast (Ultravist®, Bayer Schering Pharma AG, Berlin, Germany) and 5 mm slices with 1 mm reconstruction. The MR imaging used paramagnetic contrast (Gadovist®, Bayer Schering Pharma AG) and 1.5 mm slices. Diameters were also independently measured by an experienced radiologist on three-plane reconstructions (3D-MPR format), taking transversal sections perpendicular to the central line of the lumen.
To obtain comparable measurement by IVUS and TC, we used the principle branches as anatomical references and chose pre-established planes. In each section, we compared minimum (CTmin) and mean CT diameters (CTmean=diameter minimum+maximum diameter/2) with the minimum IVUS diameter, because the catheter might be in a noncoaxial position and the section obtained be oval and not circular (Figure 1). We rejected any measurements that were not exactly located by both methods.
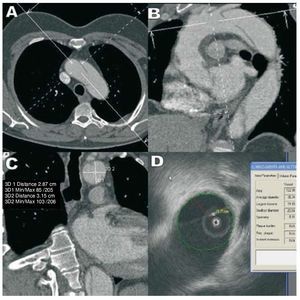
Figure 1. Measurement of the aortic lumen between the left common carotid artery terminal and the left subclavian artery in a patient with aortic dissection. A and B: computerized tomography images showing the position of the axis to measure the transversal diameter at the longest axis of the aorta. C and D: measurement of two diameters of the aortic lumen with computerized tomography (C) and of the smaller diameter with intravascular ultrasound (D).
Statistical analysis was with linear regression, Student t test for paired data and Bland-Altman analysis to calculate systematic error. To quantify selected aorta segment eccentricity, we calculated an index equal to the difference between the larger and smaller CT diameters, divided by the mean of both, and expressed as a percentage. We considered eccentricity significant when the index was >10%.
RESULTS
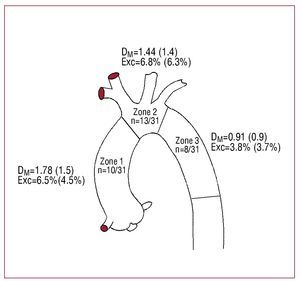
We obtained 31 comparable non-pathologic thoracic aorta segments measured by IVUS and CT (Figure 2). The mean of the diameters was: CTmin, 27.4 (9.2) mm; CTmean, 28.3 (9.8) mm; IVUS, 27.9 (9.7) mm. The correlation between methods was good (r=0.98; P<.001). The mean difference (MD) of absolute values was 1.33 (1.3) mm. Systematic error (SE) between CTmin and IVUS was 0.59 (1.78) mm (P=.077). Given that in CT, the aorta sections perpendicular to the central axis were often not circular, we also compared this with mean diameter to obtain a better approximation of IVUS and CT (SE, 0.28 [1.92]; P=.425). The difference was >/=2.5 mm between CTmin and IVUS in 19.4% (6/31) of the observations, and between CTmean and IVUS in 9.7% (3/31) (range, 0-5.1 mm). When the aorta was eccentric (eccentricity index >10%), the difference between CTmean and IVUS was greater (MD, 2.15 [1.8] mm; P=.056).
Figure 2. Diagrammatic representation of the thoracic aorta. In each zone you can see the number of measurements obtained with respect to the total, the mean difference in absolute value (SD) between the mean diameter measured by computerized tomography and the minimum diameter (DM) by intravascular ultrasound, and index of eccentricity (Exc).
DISCUSSION
The results of our study show a good correlation between linear measurements of the aortic lumen by IVUS and CT. A small but significant difference exists between the two methods with a positive bias in IVUS measurements of concentric aorta segments. This difference is less (non-significant) if we take the mean CT diameter. This could partly be explained by the fact that IVUS does not measure the true minimum diameter but, rather, a non-biased diameter.
In the other published series comparing these methods in the thoracic aorta, Fernández et al4 report a positive bias of the diameter measured by IVUS compared to the minimum diameter measured by CT, in 66% of 71 measurements. In this series, the precision of IVUS measurements increased in an inanimate model when the IVUS catheter was located on the middle line.
Other series of patients with abdominal aorta disease report substantial similarity between CT and IVUS measurements5,6 and that IVUS tends to record smaller diameters than CT. This contradiction could be explained by the curvature of the thoracic aorta. This conditions the position of the catheter, which is almost always eccentric with respect to the central axis of the vessel, giving rise to tangential measurements.
In our experience, IVUS is a safe procedure. No technique-related complications arose and IVUS enables us to visualize intraluminal defects in detail (Figure 3). In our opinion, the differences observed do not invalidate the use of IVUS in measuring the aortic lumen prior to stent implantation as they are not clinically relevant. When the diameter measured by IVUS is smaller—with the consequent risk of type I endoleaks—the difference is usually not >2 mm and almost never >10% of the normal aorta size, which is the margin of overestimation when choosing a stent.
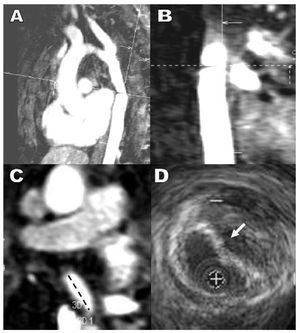
Figure 3. Post-surgical stenosis in an aortic aneurysm. Magnetic resonance sagittal (A) and coronal (B) plane views with orthogonal axes. C: maximum coarctation in axial magnetic resonance image. D: intravascular ultrasound image at the point of maximum coarctation enabling us to visualize extrinsic compression of the prosthetic tube by a parietal hematoma with no real diminution of the external caliber of the aorta.
The principle limitation of this study is that the number of patients is low, although the data obtained are congruent.
In conclusion, IVUS can be reliably used to measure aortic diameters in the concentric segments of aorta where stents are fixed. If larger series confirm these results, IVUS could be used to complement conventional imaging techniques.
Correspondence: Dr. F.J. Goicolea.
Hospital Puerta de Hierro.
Manuel de Falla, 1. 28222 Majadahonda. Madrid.
E-mail: ablasc@gmail.com
Received January 8, 2009.
Accepted for publication June 18, 2009.