Keywords
INTRODUCTION
Atherosclerosis is a systemic disease which involves large- and medium-sized arteries and commonly affects the carotid artery at its bifurcation. The prevalence of cervical internal carotid artery (ICA) stenosis greater than 50% increases from about 0.5% in people aged 50-59, to 10% in those more than 80-years-old.1 Twenty percent to 30% of patients with prior myocardial infarction or symptomatic peripheral vascular disease have >60% asymptomatic ICA stenosis.2
Carotid stenosis is an important risk factor of ischemic stroke. Approximately 10% of patients presenting with stroke have underlying carotid stenosis of 50% or more.3,4 This definition probably underestimates the contribution of carotid atherosclerosis to stroke5 but allows a standardized approach. The primary mechanism of stroke in patients with carotid artery stenosis is embolism of atherosclerotic debris or thrombotic material from the plaque into the distal cerebral vasculature. Infiltration of inflammatory cells to the surface of carotid plaques may be a critical step in promoting plaque rupture and resultant embolization, or carotid occlusion.6 Hemodynamic factors are correlated with increased stroke risk in patients with carotid stenosis.7
Cervical-artery atherosclerosis is a strong and independent predictor of myocardial infarction and vascular death; this risk increases with the degree of stenosis.8,9 In a recent population health survey, the annual death rate (mainly from cardiovascular disease) was 3.4% in patients with carotid stenosis detected by ultrasound screening compared with 1.2% in subjects without stenosis.8,9 The aims of prevention are to limit the progression of atherosclerosis and to decrease the risk of stroke and other vascular events. Therefore, carotid revascularization is only a part of the treatment of patients with carotid artery disease, which also includes strict management of risk factors and antiplatelet therapy. Carotid revascularization mainly involves carotid endarterectomy (CEA). As compared with CEA, carotid angioplasty and stenting (CAS) avoids the need for general anesthesia and an incision in the neck that could lead to nerve injury and wound complications. The costs may be less than those of surgery, mainly because the hospital stay is shorter. However, stenting also carries a risk of stroke and local complications, and the long-term efficacy of this technique is not well known. CAS is still being evaluated in the setting of randomized clinical trials (RCTs). The indications for carotid revascularization mainly depend on the degree of stenosis and whether the patient has recently experienced neurological symptoms related to the carotid artery.
ASYMPTOMATIC CAROTID STENOSIS
Natural History
Longitudinal studies in patient cohorts with carotid ultrasound suggested that the risk of first-ever ipsilateral stroke increases with the degree of stenosis; the risk is about 2% yearly in patients with stenosis greater than 50%.10,11 Analysis of the natural history risk of stroke from asymptomatic contralateral stenosis in RCTs of CEA for symptomatic carotid stenosis12,13 (Table 1), or from medical arms in RCTs of CEA for asymptomatic carotid stenosis,14,15 yielded a similar annual risk of 2% in patients with 50% stenosis or greater. The risk of ischemic stroke seems to decrease in patients with a very high degree ("near occlusion") of stenosis (Table 1).12,13 suggesting that low post-stenotic flow may protect the brain from infarction by reducing the frequency of cerebral embolism.16 Strokes occurring in the territory of an asymptomatic carotid stenosis are not always caused by the carotid lesion. Indeed, data from North American Symptomatic Carotid Endarterectomy Trial (NASCET) show that almost 50% of the strokes in the territory of a greater than 60% asymptomatic carotid stenosis are due to small vessel disease ("lacunae") and cardioembolic disease.12 In addition to the degree of stenosis, a rapid progression of stenosis,17 features of unstable carotid lesions by ultrasound,18 cerebral hemodynamic impairment,19 the presence of silent brain infarcts,10 and a high frequency of micro-emboli at transcranial Doppler20,21 have been associated with an increased risk of stroke. Results of studies, however, are conflicting.
Although available information is scarce, patients who present with asymptomatic carotid occlusion have a low-risk of subsequent ischemic stroke. In a study including 30 asymptomatic patients with carotid occlusion followed for 34 months, only 1 experienced a stroke which was in another territory.5,22 Stroke in the territory of carotid occlusion seems to result mainly from compromised cerebral blood flow. Other potential mechanisms include emboli arising from the distal or proximal ICA stump, or from plaques in the common ICA, or external carotid artery. These find their way to the ipsilateral hemisphere or retina via collateral pathways involving the external carotid artery.23
Carotid Endarterectomy
Guidelines on CEA for asymptomatic carotid stenosis are largely based on the results of 2 large, randomized studies of CEA versus best medical therapy, Asymptomatic Carotid Atherosclerosis Study (ACAS),14 and Asymptomatic Carotid Surgery Trial (ACST).15 In ACAS,14 a total of 1662 patients with asymptomatic carotid artery stenosis of 60% or greater reduction in diameter were randomized. ACAS only accepted surgeons with an excellent safety record: 40% of applicants were rejected and some surgeons were excluded during the trial because of adverse operative outcomes. The aggregate risk over 5 years for ipsilateral stroke and any perioperative stroke or death was estimated to be 5.1% for surgical patients, and 11.0% for patients treated medically (aggregate risk reduction of 53% [95% confidence interval, 22%-72%]). In the ACST,15 3120 patients with over 60% mainly asymptomatic carotid stenosis (12% had symptoms at least 6 months previously) were randomized to immediate CEA plus medical treatment versus medical treatment alone or until the operation became necessary. Surgeons were required to provide evidence of an operative risk of 6% or less for their last 50 patients having a CEA for asymptomatic stenosis, but none were excluded on the basis of his or her operative risk during the trial. Selection of patients was based on the uncertainty principle, with very few exclusion criteria. The net 5-year risks were 6.4% versus 11.8% for all strokes (net gain, 5.4% [3.0-7.8]; P<.0001) and 3.5% versus 6.1% for fatal or disabling strokes (net gain, 2.5% [0.8-4.3]; P=.004). Thus, despite differences in methods, the results are remarkably similar. The absolute reduction in 5-year risk of stroke (or any operative death) with surgery was about 5%, whereas the absolute reduction in 5-year risk of disabling or fatal stroke was about 2.5%. About 100 patients would need to undergo the operation to prevent 1 event per year and that about 200 patients would need to be treated to avoid 1 disabling or fatal stroke per year. The main differences between the trials were in the combined operative risk of stroke and death (1.5%; 95% CI, 0.6%-2.4%; in ACAS vs 3.0%; 95% CI, 2.1%-4.0%, in ACST). While the low operative risks in ACAS may not be matched in routine clinical practice, the operative risks in ACST were similar to those reported in a recent systematic review of surgical case series.24
It should be stressed that, even in the optimal environment of clinical trials, the absolute risk reduction of stroke with CEA was only about 1% per year. The crossover of the Kaplan-Meier curves occurs between 1 and 2 years after surgery and the benefit of CEA becomes significant only after 5 years of follow-up. Therefore, CEA should not be performed in centres with surgical complication rates >3% or in patients with less than a 5-year life-expectancy.
As the overall benefit of CEA is marginal in asymptomatic patients, specific subgroups of patients with a higher benefitrisk ratio of surgery need to be identified. In a pooled analysis of the data from ACAS and ACST,24 there was clear evidence for benefit from surgery in men, but not in women at 5 years follow-up, although some benefit may accrue with longer follow-up in ACST.24 Benefit from CEA in asymptomatic patients over the age of 75 years is unknown. Patients over the age of 80 years were excluded from ACAS.14 No significant net benefit of successful CEA was found among the 650 patients older than 75 years of age randomized in ACST.15 This finding might well be a false negative result. However, these patients have a short life expectancy (half of them die within 5 years from unrelated causes) and any net benefits would probably be of limited duration.
In contrast to trials of CEA in patients with symptomatic carotid stenosis, neither ACST15 nor ACAS14 showed increasing benefit from surgery with increasing degree of stenosis within the 60%-99% range. Other potential approaches to identify patients at high risk of stroke and more likely to benefit from surgery include transcranial Doppler to identify patients with microembolic signals,21 hemodynamic assessment to identify patients with inadequate collateral supply,7 and imaging methods to identify unstable atherosclerotic plaques.25
Carotid Stenting
There is no data to support the preferential use of CAS over CEA in asymptomatic patients that are good surgical candidates. The only randomized trial26 has been limited to patients at high risk for surgical complications. Any conclusion regarding efficacy of CAS compared to CEA can be drawn from nonrandomized registries.27,28 Regarding safety, periprocedural stroke and death rates in these registries were over 3%, a complication rate that would obviate any benefit with CAS for asymptomatic patients, based on the rates observed in the ACAS and ACST surgical trials.
CAS has been generally limited to patients with perceived high risk for perioperative morbidity and mortality, despite very little evidence supporting its value. Factors commonly associated with higher surgical risk have included octogenarians, significant cardiac, pulmonary, or renal comorbidities, and anatomic factors such as surgically inaccessible lesions, a stenosis that recurred after CEA or was caused by radiation therapy, contralateral stenosis, or occlusion. Many of these factors were exclusion criteria for the large surgical endarterectomy trials. It must be stressed that these factors are associated with a higher risk for complications from CEA, not a higher risk for stroke on medical therapy. The natural history on medical therapy for patients with asymptomatic carotid stenosis and risk factors for a poor surgical outcome is unknown.27,28
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study26 was a randomized noninferiority trial comparing CEA to CAS in patients at high risk for surgery. There was no medical therapy control group. Most patients (71%) were asymptomatic (≥80% stenosis). The cumulative incidence of death, stroke, or myocardial infarction within 30 days after the intervention or death, or ipsilateral stroke between 31 days and 1 year occurred in 12.2% of patients randomly assigned to undergo CAS and in 20.1% of those randomly assigned to undergo CEA, leading to the conclusion that among patients with severe carotid-artery stenosis and coexisting conditions, carotid stenting with the use of an emboli-protection device is not inferior to carotid endarterectomy. One of the major issues with this trial was the absence of a medical control group, precluding any conclusion on the superiority of CEA or CAS over medical treatment alone, in this particular population. If the risk of stroke on medical therapy in these patients at high risk for surgery is similar to the stroke risk in patients that are good surgical candidates, there would be no benefit with carotid revascularization (either by CAS or CEA), given the high rates of 30-day and 1-year outcomes in both the CAS, and CEA arms in SAPPHIRE. Therefore, the safety and efficacy of CAS and CEA in patients at high risk for surgery need to be tested against medical therapy.27,28
SYMPTOMATIC CAROTID STENOSIS
Natural History
The risk of recurrent ipsilateral stroke in patients with carotid artery stenosis is much higher than the risk of a first-ever stroke and is clearly related to the severity of carotid stenosis.29-31 In NASCET,29,30 the risk of recurrent ipsilateral stroke was 3.7% per year in patients with less than 50% carotid stenosis. This risk rose to 4.4% in patients with 50%-69% stenosis and to 13% in those with greater than 70% stenosis. However, this increased risk of stroke was present mainly during the 2 to 3 years after the first ischemic event. Then, the risk returns to a low baseline. In addition to the degree of stenosis, plaque surface irregularity is an independent risk factor for stroke.32,33 In a pooled analysis of European Carotid Surgery Trial (ECST) and NASCET,34 the risk of ipsilateral ischemic stroke fell with time since last event, rose with age, and was higher in men than in women, higher in patients presenting with hemispheric events than retinal events, and in diabetics. Other risk factors include neurological signs, severity of the atheromatous disease,35 and cerebral hemodynamic compromise.19
Patients with symptomatic ICA occlusion have an overall risk of subsequent stroke of approximately 5.5% per year and a risk of ipsilateral stroke of 2.1% per year.36 Some studies suggest that the risk of recurrent stroke is increased in patients with impaired hemodynamic measurements.36,37
Carotid Endarterectomy
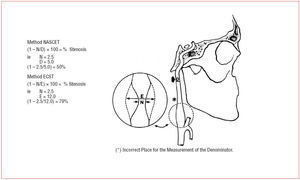
Findings from 2 large randomized, clinical trials-- NASCET and ECST30,31--have established endarterectomy as the standard treatment for severe symptomatic carotid-artery stenosis. Patients were recruited if they had had a recent carotid distribution transient ischemic attack, non-disabling ischemic stroke, or a retinal infarction, in the territory of a stenosed carotid artery. Before randomization, patients were seen by a neurologist or stroke physician to confirm their eligibility, and that the symptomatic carotid artery was imaged by selective catheter angiography. In both studies, patients were stratified according to the severity of stenosis based on catheter angiography measurements. Figure 1 shows methods of measurement of the degree of carotid stenosis on an arterial angiogram in NASCET and ECST. In both studies, the group of patients with most benefit from CEA was the group with severe stenosis (70%-99%), in whom ipsilateral fatal and disabling strokes were all significantly reduced by more than 60% over a 2-year period. The benefit became more diluted or disappeared with more moderate degrees of stenosis and in patients with near occlusions (Figure 2).
Figure 1. Methods of measurement of the degree of stenosis on an angiogram in NASCET and ECST.
Figure 2. Effect of CEA on absolute risk of ipsilateral ischemic stroke (and any operative stroke or death) at 3, 5, and 8 years' follow-up by degree of symptomatic carotid stenosis, in analysis of pooled data from ECST and NASCET. From Rothwell et al.38 ARR indicates absolute risk reduction.
In a recent meta-analysis38 of individual patients' data from the main randomized trials, using the same method of measurement of stenosis (NASCET) and definitions of outcome events, surgery increased the 5-year risk of ipsilateral ischemic stroke in patients with less than 30% stenosis (n=1746, absolute risk reduction 2.2%, P=.05), had no effect in patients with 30%-49% stenosis (n=1429, 3.2%, P=.6), was of marginal benefit in those with 50%-69% stenosis (n=1549, 4.6%, P=.04), but the benefit increased over 5 and 8 years, and was highly beneficial in those with 70% stenosis or greater without near-occlusion (n=1095, 16.0%, P<.001). There was a trend towards benefit from surgery in patients with near-occlusion at 2 years' follow-up (n=262, 5.6%, P=.19), but no benefit at 5 years (1.7%, P=.9).
Some features alter the benefit-to-risk ratio for CEA for moderate carotid stenosis. Benefits were greatest among those with more severe stenosis, those ≥75 years of age, men, patients with recent stroke (rather than TIA), and patients with hemispheric symptoms rather than transient monocular blindness.30,39
In a pooled analysis of ECST and NASCET trials,34 benefit from surgery was greatest in men, patients aged 75 years or older, and those randomized within 2 weeks after their last ischemic event, and fell rapidly with increasing delay. For patients with 50% or higher stenosis, the number of patients needed to undergo surgery (ie, number needed to treat) to prevent 1 ipsilateral stroke in 5 years was 9 for men versus 36 for women, 5 for age 75 years or older versus 18 for younger than 65 years, and 5 for those randomized within 2 weeks after their last ischemic event, versus 125 for patients randomized after more than 12 weeks. These results were consistent across the 50%-69% and 70% or more stenosis groups, and across the 2 trials. Therefore, in patients with carotid TIA or nondisabling ischemic strokes, the procedure should be done within 2 weeks of the patient's last symptoms.34 Other radiographic factors found to predict better outcomes after CEA include the presence of intracranial stenosis, the absence of leukoaraiosis, and the presence of collaterals.30,40,41 Table 2 shows the recommendations from the American Heart Association/American Stroke Association Council on Stroke for Interventional Approaches to Patients With Stroke Caused by Large-Artery Atherosclerotic Disease.42 In a systematic review of all studies published from 1980 to 2000 that reported the risk of stroke and death resulting from CEA, the operative risk was 5.1% (4.6-5.6) for symptomatic stenosis.43
Carotid Stenting
In 2005, a systematic review44 of 5 randomized trials comparing stenting with endarterectomy concluded that the current evidence does not support a change from the recommendation of carotid endarterectomy as the standard treatment for carotid stenosis. No significant difference in the major risks of treatment was found but the wide confidence intervals indicate that it is not possible to exclude a difference in favor of 1 treatment. The 30-day risks of stroke or death were 8.1% for patients treated with CAS and 6.3% for those treated with CEA; the odds ratios for treatment-related death or any stroke after 30 days were 1.33 (95% CI, 0.86-2.04), and 1.01 (95% CI, 0.71-1.44) after 1 year. The authors recommended that patients suitable for carotid endarterectomy should only be offered stenting within the ongoing randomized trials of stenting versus surgery.
Since this time, the short-term results of the Stent-Protected Angioplasty versus Carotid Endarterectomy in symptomatic patients (SPACE)45 and Endarterectomy Versus Stenting in patients with Symptomatic Severe carotid Stenosis (EVA-3S)46 trials have been published, almost doubling the number of patients available for analysis.
EVA-3S is a French multicentre, non-inferiority randomized trial with national research organization funding to compare stenting with endarterectomy in patients with a symptomatic carotid stenosis of at least 60%.46 Patients were eligible if they had experienced a carotid TIA or nondisabling stroke within 4 months before randomization, associated with an atherosclerotic stenosis within the ipsilateral carotid bifurcation of at least 60% NASCET, which investigators believe was suitable for both carotid surgery and angioplasty. The interventional physicians had to document at least 12 cases of CAS. Carotid angioplasty initially consisted of stenting with or without the use of cerebral protection. After 80 patients had been treated in the CAS arm, the Safety Committee recommended using cerebral protection devices systematically because the 30-day rate of stroke was 3.9 (0.9 to 16.7) times higher than that of unprotected CAS. The trial was stopped prematurely after the inclusion of 527 patients for reasons of both safety and futility. The 30-day incidence of any stroke or death was 3.9% after endarterectomy (95% CI, 2.0-7.2) and 9.6% after stenting (95% CI, 6.4-14.0); the relative risk of any stroke or death after stenting as compared with endarterectomy was 2.5 (95% CI, 1.2-5.1). The 30-day incidence of disabling stroke or death was 1.5% after endarterectomy (95% CI, 0.5-4.2) and 3.4% after stenting (95% CI, 1.7-6.7); the relative risk was 2.2 (95% CI, 0.7-7.2). At 6 months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P=.02). In this study of patients with symptomatic carotid stenosis of 60% or more, the rates of death and stroke at 1 and 6 months were lower with endarterectomy than with stenting.
SPACE45 also investigated whether CAS is not inferior to CEA in patients over the age of 50 years with symptomatic (transient ischemic attack or minor stroke) stenosis (at least 70% ECST) eligible for both methods. The primary investigator of each centre had to demonstrate 25 successful interventions prior to participation in the trial, whereas secondary investigators got a preliminary certificate after 10 interventions. In all, 1200 patients were randomized between March 2001 and January 2006. Of the CAS patients 26.6% were treated using an embolic protection device. The rate of ipsilateral stroke and death between randomization and day 30 after treatment (primary endpoint) was 6.84% in the CAS and 6.34% in the CEA group. As the upper limit of the 90% confidence interval of the absolute risk reduction exceeded the predefined non-inferiority margin of 2.5%, SPACE failed to prove the non-inferiority of CAS compared with CEA in treating patients with symptomatic carotid artery stenosis. The authors concluded that the results of this trial do not justify the widespread use in the short-term of carotid-artery stenting for treatment of carotid-artery stenoses.
When comparable endpoints are used, both studies show similar results regarding the 30 day rate of stroke or death with carotid stenting : 7.7% (95% CI, 5.7%-10.1%) in SPACE compared to 9.6% (95% CI, 6.3%-13.8%) in EVA-3S, with a large overlap between confidence intervals. If we focus on the 30 day rate of severe events (ie, disabling stroke or death), it was apparently higher in SPACE (4.8%; 95% CI, 3.4%-6.8%) than in EVA-3S (3.4%; 95% CI, 1.6%-6.4%), but once again there is a large overlap between confidence intervals. The 30 day rate of stroke or death in the surgical group was lower in EVA-3S (3.9%) than in SPACE (6.5%), resulting in a larger difference between the stenting and surgery groups, which partly explains why there is a statistically significant difference in favour of surgery in EVA-3S, but not in SPACE. However, the lower 30 day rate of stroke or death in the surgical arm of EVA-3S may be due to chance, since the confidence interval of this rate (3.9%; 95% CI, 1.9%-7.0%) includes the value of 6.5% reported in SPACE.47
An updated meta-analysis48 of all studies shows a significant 41% relative increase of any stroke and death within 30 days after treatment in the endovascular treatment group compared to the surgical group (OR, 1.41; 95% CI, 1.07-1.87; P=.016). However, a significant heterogeneity was found in this analysis. Possible reasons for the heterogeneity are the facts that some trials included asymptomatic patients, and that the endovascular technique changed during the time period under consideration. There was a non significant 33% relative increase of disabling stroke and death after treatment in the endovascular treatment group compared to the surgical group (OR, 1.33; 95% CI, 0.89-1.98; P=.17). No significant heterogeneity was found for this analysis. According to present knowledge, CAS is not safer than CEA, nor does it provide a better outcome, at least in the short term (30 days). Long-term follow-up data of the SPACE and EVA-3S trials are not yet available. In particular, the rates of restenosis after surgical and endovascular treatment may have an impact on decision making--despite restenosis being asymptomatic in most cases. Patients should be informed that surgical treatment is still the standard treatment for prevention of recurrent ipsilateral ischemic stroke resulting from severe carotid stenosis.
A large number of patients will be needed to identify which factors related to patient characteristics, operator experience, and the procedure itself are associated with a high risk of stroke after CAS. In this respect, operator experience was not a major determinant of the 30-day risk of stroke or death in the EVA-3S trial. Among the patients in the stenting group, 15.8% were treated by interventional physicians who had performed more than 50 carotid-stenting procedures, 45.4% by physicians who had performed 50 or fewer procedures, and 38.8% by physicians still in procedural training. The 30-day risk of stroke or death for these 3 groups was 12.2%, 11.0%, and 7.1%, respectively (P=.49).47
In symptomatic patients considered at high risk for surgery, data from recent registries of CAS show much higher 30-day rates of stroke or death than those reported in the SAPPHIRE trial.26 The recently published multicentre registry Carotid RX Acculink/Accunet Post-Approval Trial to Uncover Unanticipated or Rare Events (CAPTURE),49 for example, reported a 30-day risk of stroke, death or myocardial infarction of 12% among 483 patients with symptomatic stenosis at high risk for surgery.
CAD and Stroke After Coronary Artery Bypass
In a systematic review of the literature from 1970 to 2000,50 the risk of stroke after coronary artery bypass graft (CABG) was 2%, with a mortality of 23%. The risk increased to 3% among patients with predominantly asymptomatic unilateral carotid stenosis of 50%-99%, 5% among those with bilateral stenoses of 50%-99%, and 7%-11% among those with carotid occlusion. Significant predictive factors for post-CABG stroke included; a) carotid bruit (odds ratio 3.6; 95% CI, 2.8-4.6), b) prior stroke/TIA (odds ratio 3.6; 95% CI, 2.7-4.9), and c) severe carotid stenosis/occlusion (odds ratio 4.3; 95% CI, 3.2-5.7). This systematic review indicated that 50% of stroke sufferers did not have significant carotid disease and 60% of territorial infarctions on CT scan/autopsy could not be attributed to carotid disease alone.
Most strokes in patients undergoing cardiac surgery, including those with carotid stenosis, are not related to hypoperfusion.50,51 In 1 study, only 9% of strokes after CABG were in watershed (hypoperfusion) areas.51 Most perioperative strokes in such patients cannot be attributed to carotid stenosis because of their location (contralateral to the affected carotid artery or bilateral). Most perioperative strokes result from manipulations of the heart and aorta, or release of particulate matter from the cardiopulmonary-bypass pump. Delayed embolism is often attributed to postoperative atrial fibrillation, myocardial infarction and hypercoagulability due to surgical trauma, and associated tissue injury. General anesthesia, dehydration, bed rest, stasis in the postoperative period, and perioperative withholding of antiplatelet or anticoagulant agents can aggravate surgery-induced hypercoagulability. Other, less common causes of perioperative stroke include air, fat, or paradoxical embolism, and arterial dissection resulting from neck manipulations during induction of anesthesia and neck surgery.52
The benefit of prophylactic carotid surgery or stenting to prevent peri-operative strokes in patients undergoing CABG or other major vascular surgery remains unproven. Revascularization before surgery is generally unwarranted, but some patients with hemodynamically significant, high-grade, asymptomatic carotid stenosis--in particular those with bilateral stenoses--may benefit from carotid revascularization before elective surgery.50-53 Another debate is whether CEA should be staged or synchronous. In a systematic review of 97 published studies following 8972 staged or synchronous operations, 10%-12% of patients undergoing staged or synchronous procedures suffered death or major cardiovascular morbidity (stroke, MI) within 30 days of surgery. Overall, there was no significant difference in outcomes for staged and synchronous procedures, and no comparable data for patients with combined cardiac and carotid disease not undergoing staged or synchronous surgery.54 Randomized clinical trials are needed.
CONCLUSION AND FUTURE DIRECTIONS
Although CEA is highly beneficial in patients with symptomatic severe (70% or more) carotid stenosis, it is of marginal benefit in patients with asymptomatic or symptomatic but moderate carotid disease. Consequently, specific subgroups of patients with a higher benefit risk ratio of surgery need to be identified. Characterization of plaques in terms of morphology (eg, fibrous cap rupture) and inflammatory constituents by modern imaging techniques (eg, high-resolution MRI), identification of clinically silent emboli by transcranial Doppler and of inadequate collateral supply by hemodynamic assessment, and biological markers may help identify unstable plaques and patients at high risk of first or recurrent stroke and allow the development of effective preventive and therapeutic measures.
Current data do not support the preferential use of CAS over CEA in patients that are good surgical candidates. In those that are not good surgical candidates, any revascularization procedure must be proven superior to medical therapy alone. Several RCTs are in progress or are being organized to compare CAS with CEA in patients at low or high risk for surgery and with symptomatic or asymptomatic carotid stenosis. CAS must be limited to these trials designed to provide this proof of efficacy and participation to these trials must be encouraged. Nonrandomized registry data will not provide any useful information to help guide therapeutic decisions.
Among several other important areas for further investigation pertaining to CEA, the benefits and risks of CEA in octogenarians, the management of coexisting carotid and coronary artery disease, and whether improved medical treatment for atherosclerosis (eg, statins) may erase the small benefit of CE in patients with asymptomatic stenosis or 50% to 69% symptomatic stenosis need to be further explored.
ABBREVIATIONS
CAS: carotid angioplasty and stenting
ACAS: Asymptomatic Carotid Atherosclerosis Study
CABG: coronary artery bypass graft
CEA: carotid endarterectomy
ECST: European Carotid Surgery Trial
ICA: internal carotid artery
RCTs: randomized clinical trials
Section Sponsored by Laboratorio Dr Esteve
Correspondence: Prof. Jean-Louis Mas,
Service de Neurologie, Hôpital Sainte-Anne,
1 rue Cabanis, 75674 Paris Cedex 14, France,
E-mail: jl.mas@ch-sainte-anne.fr