Keywords
INTRODUCTION
Atherosclerosis is a diffuse systemic disease that is characterized by the existence of lipid-rich plaques in the walls of large and medium-sized arteries. Plaques with high lipid content have been referred to as "unstable", "vulnerable," or "high-risk" because of their propensity to rupture, thus predisposing the patient to an acute event. On the clinical level, atherosclerosis is basically characterized by the formation of an acute (occlusive) thrombus anchored to a ruptured or eroded plaque; thus, the term atherothrombosis is a more complete definition of the nature of the disease. Despite the fact that, currently, atherosclerotic disease is usually diagnosed once the local signs (acute coronary syndrome, transient ischemic attack [TIA], acute stroke, or limb ischemia) have appeared, due to the generalized nature of the disease, it can be diagnosed in the preclinical or asymptomatic stage, a circumstance that enables early treatment. Atherothrombosis is the major cause of morbidity and mortality in developed countries, and the future is not much more promising, either in developed or developing countries. Ever since Virchow proposed his hypothesis concerning the development of atherosclerosis, obstructive atherosclerotic disease has been divided into three categories: coronary artery disease, cerebrovascular disease, and peripheral arterial disease.
Based on a common origin, all these clinical manifestations are currently grouped under the single term "atherothrombotic disease."
PATHOPHYSIOLOGY
From the pathophysiological point of view, atherosclerosis is a single systemic disease caused by a common process, regardless of the vascular territory involved.
Different studies demonstrate that evidence of atherosclerosis can be detected as early as the second or third decade of life, prior to the onset of clinical signs.1-3 Atherosclerosis begins with the dysfunction of the endothelium (the natural barrier between the blood flow and the arterial wall). Its main function is to render the wall "nonadhesive" and capable of adapting to the rheological needs of the arterial vessel. The initial lesions are fatty streaks, which consist of the subendothelial accumulation of cholesterol-loaded macrophages (foam cells) that appear very early in the aorta, subsequently in the coronary arteries and, later, in the cerebral circulation.4 Once the endothelium becomes dysfunctional, it allows low-density lipoprotein (LDL) cholesterol molecules to penetrate the arterial wall through the endothelium. In an attempt to contain this "invasion", monocytes/macrophages phagocytose the previously oxidized LDL cholesterol and become cholesterol-loaded cells. When the macrophages are replete with cholesterol, they become foam cells. Due to the fact that cholesterol is not easily metabolized outside the liver, its continuous accumulation within the cells, which fail to thrive, leads them to commit "suicide" (apoptosis or programmed cell death),5 with the resulting release of prothrombotic substances, such as tissue factor. Following the death of the macrophage foam cells, the cholesterol is again released into the arterial wall, thus perpetuating the process.6,7 Finally, the cholesterol can crystallize, a factor that has recently been reported to destabilize the plaque.8
Endothelial dysfunction is a reversible systemic disorder that is considered to be the earliest disease process involved in atherosclerosis.9,10 It is the failure of the endothelium as a natural barrier, which not only promotes incompetence at the functional level, but permits the infiltration of the arterial wall by different harmful substances, including the recruitment of inflammatory cells in the vessel wall.
Shear stress also plays a fundamental role not only in the formation of the atherosclerotic plaque, but in the differences in the composition and behavior of the plaques as well,11 even stimulating the expression of inflammatory genes and adhesion molecules by the endothelium.12
Activated endothelial cells produce cytokines and express adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). Blood cells with receptors for these adhesion molecules (lymphocytes, monocytes) bind to them and, due to the cytokines secreted, are stimulated to migrate through the endothelial junctions toward the intima, where they are transformed into macrophages, a step that is highly important in the development of atherosclerosis.14 The LDL cholesterol particles, which, under normal conditions, would be transported to the liver, undergo oxidation, a circumstance that provokes an inflammatory response and their uptake by macrophages.
As the lipid core of the atheromatous plaque grows due to the accumulation of LDL cholesterol particles and macrophages, smooth muscle cells migrate from the media toward the intima. These cells produce and secrete collagen and fibrous elements of the extracellular matrix, resulting in the formation of the fibrous covering of the fibroatheromatous plaques. Initially, these plaques do not reduce the vascular lumen because there is a compensatory dilatation of the vascular wall (positive remodeling).15 The plaque grows eccentrically, causing the thinning of the media and adventitia, until the compensatory dilatation can no longer continue, at which time, the plaque begins to grow toward the center of the vascular lumen, compromising the blood flow.
Most acute cardiovascular events are not caused by the progressive narrowing of the vascular lumen, but by complications involving the atherosclerotic plaque (rupture, ulceration, hemorrhage, erosion) that provoke acute vascular occlusion due to vessel thrombosis.
EARLY DETECTION OF ATHEROSCLEROSIS USING IMAGING TECHNIQUES
As we mentioned above, atherosclerosis can develop as early as the second or third decade of life. Moreover, the lesions that provoke the symptoms often are not stenotic and, thus, are not detectable by conventional contrast angiography. For this reason, the visualization and composition of the plaque is more important than the degree of stenosis.
Over the last few years, great advances have been made in imaging techniques that enable the visualization and characterization of atheromatous plaques, as well as the monitoring of their progression or regression (Figure 1).16 Moreover, once atherosclerosis is detected in one territory, given the diffuse nature of the disease, we can assume that all the territories are affected. Its early detection would generate novel opportunities for primary prevention through changes in lifestyle or even through drug therapy, especially in patients at high cardiovascular risk.
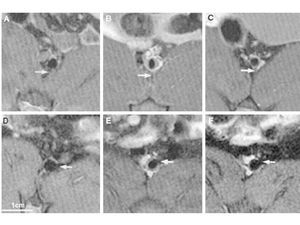
Figure 1. Serial magnetic resonance images in rabbit abdominal aorta showing progression and regression of an atherosclerotic plaque. An increase in the size of the atheromatous plaque nine months after induction of atherosclerosis, followed by its regression six months after discontinuation of the atherogenic diet. Panels A and D correspond to baseline images at different levels of abdominal aorta. Panels B and E correspond to the images obtained at these two levels, respectively, months after induction of atherosclerosis. Finally, panels C and F correspond to the images obtained 6 months after discontinuation of the atherogenic diet. Reproduced with the permission of Helft et al.15
INTIMA-MEDIA THICKNESS
The intima-media thickness is considered and has been validated as a marker of generalized atherosclerotic disease.17 In fact, it correlates linearly with the number of atherosclerotic risk factors.18
The measurement of the intima-media thickness and the quantitative and qualitative analysis of the atheromatous plaques can be performed using, among other techniques, magnetic resonance (MR) imaging and surface or transesophageal ultrasound.
B-mode ultrasound with Doppler flow measurement has been established as the imaging modality of choice for visualizing the intima-media thickness. An 8-MHz transducer can be utilized to measure this index in medium-size to large arteries, such as the carotid, femoral, or radial arteries. Likewise, it is possible to characterize the visualized atheromatous plaque since the echogenicity of the plaque is determined by its composition: a heterogeneous hypoechoic plaque is associated with the presence of lipids, whereas a homogeneous hyperechoic plaque is mainly fibrous.19
Different studies in which ultrasonographic measurements were compared with the histological findings have demonstrated that the determination of the intima-media thickness of posterior wall of the carotid artery by means of ultrasound reflects the true thickness of the wall,20-22 although it appears that the measurements recorded with ultrasound may be slightly greater. The values provided by the measurement of proximal carotid wall are less accurate.
Although a number of measurements of differing complexity can be carried out, such as calculating the mean of 12 different points along the carotid artery or the inclusion of the measurement of the carotid bifurcation, the measurement most frequently employed is that of common carotid artery23,24 for two reasons: because it is the most reproducible approach and because it has been shown to have a capability for predicting events similar to that of more complex and complete methods. However, the combination of information from different segments can increase the accuracy of this index.
It must be kept in mind that, since the intima-media thickness is a continuous variable, there is no clear upper cut-off value with which to define an atherosclerotic plaque. Normal carotid intima-media thickness has been arbitrarily established as 0.5 mm to 1.2 mm25; thus, values over 1.1 mm to 1.2 mm are considered to define the presence of an atherosclerotic plaque.
One of the premises on which the use of this index as a vascular marker is based is that it indicates generalized atherosclerosis. Increased carotid intima-media thickness reflects atherosclerosis in the abdominal aorta26 and in the arteries of the lower limbs,27-29 and has even been reported to be associated with increased left ventricular mass.30,31 With respect to coronary artery disease, a number of studies have demonstrated that atherosclerosis in the carotid artery and aorta are markers of coronary atherosclerosis.32-35 Patients with symptomatic coronary artery disease have an increased carotid intima-media thickness in comparison with asymptomatic controls.36 Carotid wall thickening has also been found in patients with silent ischemia.37 In any case, although the relationship between carotid intima-media thickness and the severity of coronary artery disease is quite common, it is important to point out that it is weak.38
The intima-media thickness is a marker not only for coronary atherosclerosis, but for the progression of coronary artery disease as well, as was seen in prospective epidemiological studies.17 The Cardiovascular Health Study found that an increase in the intima-media thickness increased the relative risk of acute coronary syndrome or acute stroke in patients over 65 years of age.34 In prevention trials involving lipid-lowering treatments, it was observed that a decrease in intima-media thickness was associated with a reduction in the incidence of cardiovascular events.39-42 Thus, given that it correlates well with the progression/regression of atherosclerosis, the measurement of the intima-media thickness of the carotid artery may be a good method for monitoring the effect of treatment in patients.
IMPORTANCE OF CALCIUM AND ITS CORRELATION WITH THE ATHEROSCLEROTIC BURDEN
The identification of coronary artery calcium is an indicator of chronic atherosclerotic changes in the coronary artery wall.43 Coronary artery calcification is a common phenomenon that is not necessarily indicative of obstructive coronary artery disease. In most cases, it is a sign of chronic changes in arterial wall remodeling and, less frequently, of vulnerable or high-risk plaques. However, it is known that the more widespread the chronic atherosclerotic involvement, the stronger the association with vulnerable or high-risk plaques and, thus, with a higher incidence of ischemic events.
At the present time, there are two techniques for quantifying coronary artery calcium: electron beam computed tomography and multidetector (multislice) computed tomography (MDCT). Both techniques are capable of accurately quantifying the coronary artery calcium load. Although the electron beam technique is considered the "gold standard" for this determination, the approach most widely used for the quantification of coronary artery calcium is multislice computed tomography due to its greater availability and its good correlation with the electron beam technique; in addition, its reproducibility is better.44,45
The technique described by Agatston et al46 consists of measuring the total area of the calcified coronary plaque in pixels, slice by slice, and assigning it a score. The Agatston calcium score is obtained as a result of multiplying the area of the calcified lesion by a factor that depends on the peak attenuation in the lesion. There can be problems with noise artifacts and variations in the scanning protocol (mainly due to differences in slice thickness), which lead to variations in the reproducibility. For this reason, new approaches for calculating calcium volume and mass that exhibit greater reproducibility and consistency have recently been designed.47
The calcium score provides an accurate estimation of the coronary atherosclerotic burden and is a powerful predictor of cardiac events in asymptomatic patients.48 Patients with no coronary calcium or a low calcium score have of lower probability of experiencing clinical events than those with high scores.49 Along general, consensus-based lines, the coronary atherosclerotic burden is considered to be minimal (calcium score of 0 to 10), mild (calcium score of 11 to 100), moderate (calcium score of 101 to 400) or severe (calcium score over 400). A calcium score over 1000 is associated with an annual risk of experiencing a cardiovascular event of 25% (indicating the need for aggressive therapeutic measures in these patients). However, these values have to be adjusted according to age and sex, on the basis of the percentiles reported for the general population, since a calcium score of 45 in a 45-year-old man (indicating moderate risk as it is above the 95th percentile for that age) is not the same as a score of 45 in a 75-year-old man (who would be below the 10th percentile).50
Interestingly, ethnic differences in the atherosclerosis-related calcium burden have also been reported, and can not be explained by the known conventional risk factors.51
It is important to point out that the coronary calcium score has not been shown to be a predictor of events independently of the conventional risk factors.50 For the stratification of the risk of cardiovascular events, algorithms that combine the classical risk factors, such as the Framingham study or the Prospective Cardiovascular Munster (PROCAM) study are generally employed. Conference V on the prevention of cardiovascular disease, sponsored by the American Heart Association,52 addressed the use of the calcium score, among other noninvasive tests, in selected populations of asymptomatic patients, and it has been endorsed by the National Cholesterol Education Program (NCEP, Panel III).53 For example, asymptomatic patients with an intermediate risk (ten-year risk of 10% to 20% and two or more coronary risk factors according to the Framingham criteria) constitute the group that could benefit the most from a change in treatment and lifestyle and, thus, would be the group in which the quantification of the calcium score would be most clearly indicated. There would also be certain subgroups of selected low-risk patients, for example, young patients with a family history of early-onset ischemic heart disease, that could benefit from this technique.54 In these patients, a high calcium score would markedly increase the risk of cardiovascular events, and measures aimed at prevention would need to be intensified. If, on the other hand, the results of the test were negative, the risk would be lower. Some authors indicate that the combination of the calcium score with the conventional risk factors would be a better approach to estimating risk in asymptomatic patients.55,56 Moreover, in those patients with cardiovascular risk factors and a high calcium score, stress perfusion imaging and/or a ventricular function test can provide diagnostic and prognostic information.56
Recent data indicate that, at the present time, serial monitoring involving the calcium score to observe the progression or regression of atherosclerosis is not recommended.54,57
NEW IMAGING TECHNIQUES FOR QUANTIFICATION OF THE OVERALL ATHEROSCLEROTIC BURDEN
The imaging of atherosclerotic plaques has traditionally focused on assessing the degree of stenosis that the plaques produce in the arterial lumen. However, as was mentioned above, it is now a well known fact that most cardiovascular events are secondary to nonstenotic plaques.15 This demonstrates the need for imaging techniques capable of identifying not only the degree of stenosis associated with the atheromatous lesions, but their composition and degree of inflammation as well, in order to identify possible high-risk (vulnerable) plaques prior to their clinical manifestation. These imaging techniques include invasive approaches such as intravascular ultrasound, angioscopy, optical coherence tomography; and noninvasive approaches like MR, positron emission tomography (PET) and computed tomography (Table). Here we will focus only on the noninvasive techniques, with special attention to molecular imaging techniques.
Magnetic resonance is a technique that enables us to evaluate in detail the arterial vascular tree at practically every level, including coronary arteries, the aorta and its major branches, carotid arteries and the arteries of the lower limbs, and is harmless to the patient. It has been proposed as a technique that makes it possible to visualize the composition of the plaque, based on differences in the biophysical and biochemical properties of the different components of the plaque.58 By combining multicontrast sequences, it is possible to determine the anatomy of the plaque and its composition.
Contrast-enhanced MR angiography is a highly sensitive and specific technique as compared to conventional x-ray angiography for the detection of stenosis of more than 50%.59 With this approach, it is possible to perform whole body angiography, with the exclusion of intracranial and coronary arteries, which are the only territories that require site-specific studies.
Magnetic Resonance Study of Carotid Plaques
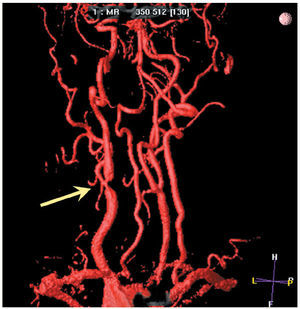
The carotid arteries are vessels of good caliber that are not subjected to movement. Thus, they are ideal for noninvasive imaging studies. Magnetic resonance makes it possible not only to quantify the size of the atherosclerotic plaque, but to assess the integrity of the fibrous cap.60 There is a close association between the thinning or rupture of the fibrous cap of the carotid plaque detected by MR and a recent history TIA or acute stroke.61 It is technically possible to combine MR angiography (quantification of the degree of stenosis and its spatial distribution) with high-resolution MR (characterization of the arterial wall and the composition of the plaque)62 (Figure 2).
Figure 2. Magnetic resonance angiographic study in a patient with neurological symptoms, showing stenosis at the right carotid artery bifurcation.
Magnetic Resonance Study of Aortic Plaques
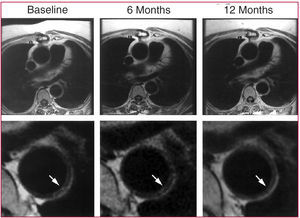
Atheromatous plaques in the thoracic aorta can be evaluated using high-resolution (submillimetric resolution) MR, although there are potential artifacts secondary to breathing movements. Magnetic resonance is more reliable than transesophageal ultrasound with respect to the characterization of the plaque, although the findings with both techniques have been shown to have a strong correlation for plaque thickness.63 It is also possible to carry out contrast-enhanced MR angiography of the thoracoabdominal aorta and its branches, which provides spatial information on the distribution of the plaques and on renal artery involvement.16 Magnetic resonance is the ideal tool for monitoring plaque regression following the initiation of lipid-lowering treatment, as has been demonstrated by our group64,65 (Figure 3).
Figure 3. Regression of atherosclerotic plaque in human descending thoracic aorta after initiation of lipid-lowering treatment. Image reproduced with the permission of Corti et al.54
Molecular Imaging Magnetic Resonance
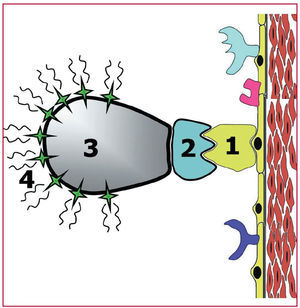
Above, we described the different imaging techniques that can be employed to detect advanced-stage atherosclerotic disease for the purpose of quantifying the size and composition of the atherosclerotic plaque. However, novel imaging modalities (molecular imaging techniques) have been developed that are potentially capable of detecting the disease in the early stages. Molecular imaging can be considered an in vivo equivalent to immunohistochemical techniques. The purpose is to identify different molecules and/or cells by means of radiolabeled markers, which enhance the signal at any site at which they are deposited. The contrast agent should identify the target or objective, exhibiting high specificity as well as a signal intense enough to be detected and differentiated from unlabeled zones66 (Figure 4).
Figure 4. Illustration of the concept of molecular imaging. The specific molecule that is to be investigated (specific receptors, integrins, adhesion molecules, etc, indicated in the Figure by the number 1) is identified by a ligand, which binds to the molecule to be studied. The ligands (number 2 in the Figure) are usually monoclonal antibodies that specifically recognize the molecule to be studied. Previously (in vitro), the ligand has been bound to signal transmitting substances (number 4 in the Figure). This bond is mediated by a vehicle (number 3 in the Figure) that is capable of binding specifically to the ligand and the signal transmitting substances. This makes it possible to locate certain molecules by means of imaging techniques.
A number of techniques have been developed with sufficient spatial resolution to afford molecular imaging: MR with targeted contrast agents, ultrasound probes, optical imaging techniques (fluorescent and bioluminescent) and nuclear medicine techniques (PET and single photon emission computed tomography [SPECT]).
Magnetic resonance offers excellent spatial resolution, although its sensitivity is lower than that of nuclear medicine techniques.66 The contrast agents can bind to monoclonal antibodies or specific peptides.67 The best results are obtained with nanoparticles that combine a high binding affinity for the target zone with the capacity to transport a sufficient amount of a paramagnetic contrast agent. The most widely employed nanoparticles are liposomes and fluorocarbon-based lipid emulsions. Molecular imaging techniques have been used for the visualization of thrombi (by means of contrast agents that bind to antibodies against fibrin, platelet *vß3 receptors), of angiogenesis (with contrast agents targeted against *vß3-integrin, which is expressed by endothelial cells only during angiogenesis)68 or of inflammation (using contrast agents that bind to antibodies targeted against antigens present during inflammatory processes, such as VCAM 1, ICAM 1 and E-selectin).69-72 Moreover, in rabbit models, it has been reported that paramagnetic iron oxide particles (USPIO) can be "attracted" by macrophages (cells that predominate in "vulnerable" plaques).74 This same observation has been made in human carotid arteries where, after the administration of USPIO, there is an accumulation of the contrast agent in the macrophage-rich zones of unstable plaques.75
Positron Emission Tomography and Single Photon Emission Computed Tomography
By bonding to different compounds radioactive isotopes (radionuclides) are capable of detecting different molecular and cellular processes and, thus, provide a useful imaging technique that enables us to identify different components of the atheromatous plaques. The radioisotope must be attached to a tracer substance prior to its administration. Isotope detection is performed mainly by PET and SPECT. The data obtained are processed and reconstructed by computer, resulting in two-dimensional and three-dimensional images and the localization of the distribution of the tracer in the tissues being studied.76 An ideal tracer for imaging atheromatous plaques should attach to components of the plaque, after which it should undergo rapid washout to allow sufficient contrast between the plaque and the blood. We briefly discuss the different target regions of atheromatous plaques on which radionuclides can be deposited to provide information on the composition of the plaque.
Matrix Metalloproteinases
Matrix metalloproteinases are proteins that "digest" the extracellular matrix of the atheromatous plaque, a circumstance that can destabilize the plaque. Their activity is considered to be an important marker of plaque vulnerability. In studies using models of atherosclerosis in mice77 and rabbits,78 the contrast agents targeted against these proteins were deposited in complicated plaques. Although it is necessary to point out that these imaging techniques are a long way from being applicable in humans, their potential for future clinical use is enormous.
Visualization of Apoptosis in the Atheromatous Plaque
Apoptosis plays an important role both in the genesis of the atheromatous plaque and in its destabilization and rupture. In apoptosis, the cells form the so-called apoptotic bodies, which are small molecules "covered" by phosphatidylserine, for which annexin V exhibits a high affinity. There are a number of radiotracers for labeling annexin V, and animal studies showing uptake of these compounds by certain atherosclerotic plaques have been reported.78 They have also been tested in humans, in whom the uptake of technetium-99m-labeled annexin V by carotid plaques was observed in patients with recent TIA.79 Endarterectomy specimens from these patients revealed the presence of the tracer in plaques with a high macrophage content. This field offers enormous potential, which, nevertheless, will need to be expanded.
Positron Emission Tomography Imaging of Plaque Macrophages
Positron emission tomography has an advantage over SPECT in that it provides better spatial resolution (4 mm to 5 mm vs 1 cm to 1.5 cm). The images obtained with PET derive from the detection of positron emission by certain radionuclides such as 11C and 18F. Thus, PET offers greater potential for the visualization of atherosclerotic plaques. Deoxyglucose is a compound that "competes" with glucose as an energy substrate for metabolically active cells, there being an incremental accumulation depending on the level of metabolic activity of these cells. The combination of 18F with deoxyglucose (FDG) is highly useful in the "visualization" of cellular metabolic activity by means of PET. This technique has been widely utilized in the detection of tumors and in the assessment of myocardial glucose metabolism.80 It has also been found to be useful for "visualizing" vascular inflammation.81 In addition to several reports on research in animals, a recent study in humans involving FDG-PET demonstrated that macrophages were responsible for FDG uptake.81 Moreover, a combination of PET and computed tomography in patients with TIA recently demonstrated that the plaques responsible for this event, not the contralateral plaques, exhibited a more marked uptake of FDG. Should this circumstance be confirmed, said technique could revolutionize the field of high-risk plaque detection, as well as monitoring of the effect of certain treatments (lipid-lowering drugs, etc).
Multidetector Computed Tomography
Aside from providing information on the calcium score, as we pointed out above, MDCT is capable of identifying noncalcified plaques when iodinated contrast agents are employed. It is also potentially capable of distinguishing different plaque components.83 Ex vivo studies in human coronary arteries comparing the pathological findings in the atherosclerotic plaques with the MDCT findings have demonstrated the existence of a good correlation with the different stages of plaque formation.84 Lipid-rich plaques can be differentiated from fibrous plaques and calcified plaques due to the differing attenuations, measured in Hounsfield units. Nevertheless, it is necessary to improve the spatial resolution of contrast-enhanced MDCT in order to increase its sensitivity and accuracy in classifying atherosclerotic plaques in coronary arteries, as well as in carotid arteries or aorta.85,86
CONCLUSIONS
Atherosclerosis is a systemic disease with local manifestations. Intimate knowledge of the processes involved in the genesis and progression of the disease and its local signs enables us to undertake an early in-depth diagnostic study using imaging techniques.
Different noninvasive imaging modalities have been validated for the detection and quantification of atherosclerosis in targeted territories (carotid arteries, aorta). There are indications of the existence of a strong correlation between atherosclerotic disease extent in these territories and its presence in other territories that are less accessible, except by invasive techniques (coronary arteries), suggesting that the overall atherosclerotic burden can be quantified by noninvasive means.
Imaging technologies under development may be of aid not only in the anatomical diagnosis of the disease, but in the functional diagnosis as well (molecular imaging). This field is highly promising, as it will enable us to identify sites at high risk for the development of future events.
ACKNOWLEDGMENTS
This report has been financed in part by Fundación Conchita Rábago de Jiménez Díaz, Fundación la Caixa (Borja Ibáñez, during 2005 and 2006) and the Spanish Society of Cardiology (Antonio Pinero, during 2006). Borja Ibáñez is currently fellow-elect of the Ischemic Heart Disease section of the Sociedad Española de Cardiología (2007).
Sección sponsored by Laboratorio Dr. Esteve
ABBREVIATIONS
ICAM-1: intercellular adhesion molecule 1
LDL: low-density lipoprotein
MDCT: multidetector computed tomography
MR: magnetic resonance
TIA: transient ischemic attack
VCAM-1: vascular cell adhesion molecule 1
Correspondence: Dr. J.J. Badimon.
Mount Sinai School of Medicine.
One Gustave L. Levy Place, Box 1030, New York, NY 10029, USA.
E-mail: juan.badimon@mssm.edu