Keywords
INTRODUCTION
Acute aortic syndrome is a modern term that includes aortic dissection, intramural hematoma (IMH), and symptomatic aortic ulcer. In the classic scenario, acute aortic dissection requires a tear in the aortic intima that commonly is preceded by medial wall degeneration or cystic media necrosis. In dissection blood passes through the tear separating intima from media or adventitia, creating a false lumen. Propagation of dissection can proceed in anterograde or retrograde fashion from the initial tear involving side branches and causing complications such as malperfusion syndromes, cardiac tamponade, or aortic valve insufficiency1-5.
PATHOPHYSIOLOGIC CONSIDERATIONS
Both acquired and genetic conditions share a common pathway leading to disintegrity of the intima. All mechanisms that weaken the media layers of the aorta will eventually lead to higher wall stress, which can induce aortic dilatation and aneurysm formation, or result in intramural hemorrhage, aortic dissection, or rupture. The most common risk condition for aortic dissection is hypertension, with chronic exposure of the aorta to high pressures leading to intimal thickening, fibrosis, calcification, and extracellular fatty acid deposition; extracellular matrix may undergo accelerated degradation, apoptosis, and elastolysis with eventual intimal disruption, most often at the edges of plaques.1,6-8
Genetic conditions such as Marfan's syndrome, vascular Ehlers-Danlos syndrome, annuloaortic ectasia, bicuspid aortic valve, and familial aortic dissection can also cause acute aortic syndromes. Common denominators to these genetic disorders include dedifferentiation of vascular smooth muscle cells and enhanced elastolysis of aortic wall components, leading to compromised intima and aortic dissection.9 Given this genetic predisposition, a detailed family history in patients diagnosed with acute aortic syndromes or sudden death is particularly important in assessing the need for family screening (Table 1).
Knowledge on incidence of aortic dissection in the general population is limited. Studies suggest an incidence of 2.6 to 3.5 cases per 100 000 person-years.2,10,11 In a review of 464 patients from the International Registry of Acute Aortic Dissection (IRAD), two thirds were male, with a mean age for all patients of 63 years.12
Although less frequently affected by acute aortic dissection, women were significantly older than men, with a mean age of 67 years.13 Frequent predisposing factors such as hypertension (72%), a history of atherosclerosis in 31%, and previous cardiac surgery in 18% are summarized in table 2.12-14 In the total registry, 5% and 4% of acute aortic dissections were thought to be related to Marfan's syndrome and iatrogenic causes, respectively21. Analysis of the young patients with dissection (<40 years of age) revealed that younger patients were less likely to have a history of hypertension (34%) or atherosclerosis (1%) but were more likely to have Marfan's syndrome, bicuspid aortic valve, and/or prior aortic surgery.14
CLASSIFICATION OF ACUTE AORTIC SYNDROMES
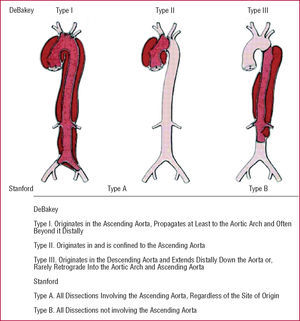
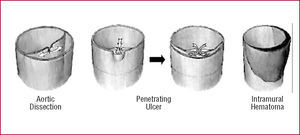
The Stanford classification of aortic dissection distinguishes between types A and B (Figure 1).15,16 In type A, the dissection involves the ascending aorta, whereas in type B only the descending aorta is involved. The deBakey classification divides dissection into 3 types, with type I dissection involving the entire aorta, type II involving only the ascending aorta, and type III sparing both ascending aorta and arch. Advanced imaging technology has defined precursors or "variants" of dissection such as intramural hematoma (IMH), penetrating aortic ulcers, and localized intimal tears (Figure 2).17-22
Figure 1. The most common classification systems of thoracic aortic dissection: Stanford and deBakey.
Figure 2. Schematic of aortic dissection (left), penetrating ulcer (middle), and intramural hematoma (right).
Classic Aortic Dissection
Acute aortic dissection is characterized by the rapid development of an intimal flap separating true and false lumen.2,23-25 The dissection can spread from the intimal tear in an antegrade or retrograde fashion, often involving side branches and causing malperfusion, tamponade, or valvular regurgitation.2,26-28 Spontaneous false lumen thrombosis (better prognosis), evidence of persistent flow communication, and/or a patent false channel (worse prognosis) may be useful to estimate late risk of expansion.5,25,29,30
Intramural Hematoma (IMH)
Aortic IMH is considered a precursor of dissection, originating from ruptured vasa vasorum in medial wall layers and resulting in an aortic wall infarct that may provoke a secondary tear with classic dissection (Figure 2).20,21 Similar to classic dissection IMH may extend, progress, regress, or resorb in up to 10 percent.19,20,31-34 IMH is frequently located in the descending aorta and associated with hypertension.35-37 Although clinical manifestations of IMH resemble dissection, IMH tends to be more of a segmental process; therefore, radiating pain to head or legs is uncommon. Nonetheless, the diagnosis of IMH depends on tomographic imaging diagnosis in the appropriate clinical setting.
Penetrating Atherosclerotic Ulcer
Deep ulceration of atherosclerotic aortic plaques can lead to IMH, aortic dissection, or perforation.38-42 Noninvasive imaging has further elucidated this entity that often further complicates IMH and appears as an ulcerlike projection into the hematoma. In association with IMH, limited series have reported seeing penetrating atherosclerotic ulcers almost exclusively in patients with type B IMH.42 Symptomatic ulcers with signs of deep erosion are prone to rupture. For these patients endovascular stent grafting is emerging as an attractive therapeutic modality.
NATURAL HISTORY AND PROGNOSIS
Type A (Proximal) Dissection
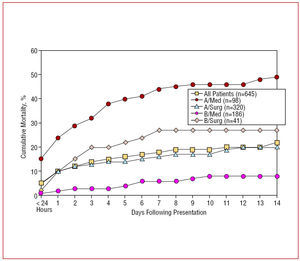
Acute aortic dissection of the ascending aorta is frequently lethal, with mortality hovering at 1% to 2% per hour early after symptom onset.23,43 The risk of death is increased in patients with complications such as pericardial tamponade, involvement of coronary arteries causing or malperfusion of the brain.2,26,28,30,44 Other predictors of increased in-hospital death include age >=70 years, hypotension, kidney failure, and pulse deficits (Table 3).28 Less appreciated predisposing factors for type A dissection include prior cardiac and valvular surgery (15%) and iatrogenic dissection from cardiac surgery or catheterization (5%). Iatrogenic aortic dissection carries a mortality slightly higher than noniatrogenic (35% versus 24%).1,12,45-48 Data from the largest registry of acute aortic dissection showed that in the absence of immediate surgical repair, medical management is associated with a mortality of nearly 24% at day 1, 29% at 48 hours, 44% at day 7, and 50% after 2 weeks. Even with surgical repair, in-hospital mortality rates are 10% after 1 day, 12% at 2 days, and nearly 20% at 2 weeks (Figure 3). The most common causes of death are aortic rupture, stroke, visceral ischemia, cardiac tamponade, and circulatory failure.28,49,50
Figure 3. Fourteen-day mortality in 645 patients from the IRAD registry stratified by medical and surgical treatment in both type A and B aortic dissection.
Type B (Distal) Dissection
Acute aortic dissection affecting the descending aorta is less frequently lethal. Patients with uncomplicated type B dissection have a 30-day mortality of 10% (Figure 3).12 However, patients with complications such as renal failure, visceral ischemia, or contained rupture often require urgent repair, with a mortality of 20% at day 2 and 25% at 1 month. Similar to type A dissection, advanced age, rupture, shock, and malperfusion are important independent predictors of early mortality.13,26,51 Chronic use of crack cocaine appears to predispose patients to acute aortic dissection with a predilection to the descending aorta.52,53
Intramural Hematoma
Proximal location of IMH is clearly considered an independent predictor of progression to dissection, contained rupture, or aneurysm formation independent of age, sex, hypertension, Marfan syndrome, bicuspid aortic valve, or local extent and diameters of IMH.33 Considering a 30 day mortality rate of 20% our findings are supported by the global experience in 456 cases of IMH with an early death rate of 16%.31,32,54-56 With timely surgical repair, however, proximal (type A) IMH is no longer related to early death (Figure 4).18,33 The high risk of "wait and see" in type A IMH, though, is reflected in 55% early mortality with medical treatment compared to 8% with surgical repair (P=.004). Considering a 12% early mortality after surgery, and a 24% death rate with medical treatment, global experience from the International Registry of Aortic Dissection (IRAD) confirms a trend of improved outcome after surgery of proximal IMH (P=.12).18,33
Figure 4. Mortality rates from intramural haematoma: overall mortality within one year in relation to medical or surgical treatment.
Interestingly, Asian patients were quoted with low death rates in proximal IMH even without surgery.32,54,56,57 However, 10 of 22 patients with type A IMH (45%) underwent surgical repair and four cases with medical management developed cardiac tamponade.58 Similarly, tamponade was observed in two of three patients with type A IMH surviving medical treatment.32 Nishigami and colleagues reported on eight medically treated type A IMH with seven survivors; yet IMH requiring surgery, such as cases with aortic diameters of >50 mm or cardiac tamponade were excluded from this analysis. Similarly, in Korean patients with type A IMH treated medically, only one of 18 patients died, four patients required pericardiocentesis or surgery for proximal dissection.14 Finally, 54% of cases with aortic diameters <50 mm eventually progressed to dissection or rupture, challenging the Asian experience that IMH in a normal sized aorta precludes progression. Thus, even Asian experience in fact underlines findings in the European gene pool that proximal IMH frequently leads to serious complications.
Actuarial survival analysis of IMH confirm better long-term outcome on oral β-blocker treatment (95% vs 67% in patients without β-blocker treatment; P=.004) as summarized in Table 5. β-adrenergic blocking agents protect by reducing wall stress, systolic arterial pressure, and the rate of pressure changes and, presumably, by stabilising the extracellular vascular matrix of the aorta.53,59 The observation that older age (>55 years) at initial diagnosis of IMH has a better long term prognosis may be explained by more focal microscars along the aortic wall inherently limiting the longitudinal progression of IMH.18,31 Accordingly, favourable outcomes of IMH are consistently reported in patients beyond 65 years.32,54,56 Thus, considering both advanced aortosclerosis with older age and the lower risk of progression, a conservative strategy (with β blockade and serial imaging) may be justified in elderly multimorbid patients and in distal IMH.33
Penetrating Aortic Ulcers
Ulcer-like projections in aortic segments of IMH identify a subset of patients at high risk. Penetrating atherosclerotic ulcers (PAU) are known to result from progressive erosion of atheromatose mural plaque penetrating the elastic lamina, finally separating media layers and setting the stage for adjacent intramural haematoma with either local or longitudinal progression. PAUs are preferentially (>90%) observed in IMH of the descending aorta, while IMH without PAU is more frequently present in the ascending aorta. Symptomatic PAU infers complications such as formation of aneurysm, pseudoaneurysm, and dissection, or unpredictable rupture. Careful imaging is vital to identify both diameter and depth of ulcers with IMH, since width >2 cm and depth >1 cm may herald the need for interventional or surgical repair to avoid rupture and death.33,42
THERAPEUTIC MANAGEMENT OF ACUTE AORTIC SYNDROME
Patients with aortic dissection typically present with the cataclysmic onset of chest and/or back pain of blunt, severe, and sometimes radiating nature. However, in contrast to classic teaching, tearing, ripping, or migratory were not common descriptors of pain in IRAD; rather, the sudden onset of severe, sharp pain was the single most common presenting complaint. Furthermore, 4.5% of patients denied any pain on presentation. Chest pain was significantly more common in patients with type A dissections (79% vs 63% of type B dissections), whereas both back pain (64% vs 47%) and abdominal pain (43% vs 22%) were significantly more common in type B dissection.12 Hypertension is the most common risk condition associated with aortic dissection, although it is less commonly present at the time of presentation, particularly in patients with type A proximal dissections (36% vs 70% for type B dissection). Acute dissections involving the ascending aorta are considered surgical emergencies. In contrast, dissections confined to the descending aorta are treated medically unless the patient demonstrates progression of dissection, intractable pain, organ malperfusion, or extra-aortic blood.
Initial Medical Therapy
The primary objective is to normalize blood pressure and initiate anti-impulse medication thus reducing the force of left ventricular ejection (dP/dt), which are main determinants of extension and rupture of the false lumen. For most patients, a blood pressure between 100 and 120 mm Hg with a heart rate of 60 bpm is attainable by titrating intravenous β-blocking agents. In patients with potential intolerance to β-blockers (those with asthma, bradycardia, or signs of heart failure), short-acting esmolol seems to be a reasonable choice. Pain and pressure control can be achieved wit h morphine sulfate and intravenous β-blockers (metoprolol, esmolol, or labetalol) or in combination with vasodilating drugs. Intravenous verapamil or diltiazem may also be used, if β-blockers are contraindicated. Monotherapy with β-blocking agents may be adequate to control mild hypertension, and in concert with sodium nitroprusside at an initial dose of 0.3 µg/kg per minute, is often effective in a severe hypertensive state. In normotensive or hypotensive patients, careful evaluation for loss of blood, pericardial effusion, or heart failure (by cardiac ultrasound) is mandatory before administering fluids. Patients with profound hemodynamic instability often require intubation, mechanical ventilation, and urgent bedside transesophageal echocardiography (TEE) or rapid computed tomography for confirmatory imaging. In rare cases, the external ultrasound diagnosis of cardiac tamponade may justify immediate sternotomy and surgical access to the ascending aorta to prevent circulatory arrest, shock, and ischemic brain damage. Percutaneous pericardiocentesis as a temporizing step has often failed, and can accelerate bleeding and shock.60
Ascending (Type A) Aortic Dissection
Acute ascending aortic dissections (Stanford type A or deBakey type I or II) should be treated as a surgical emergency because these patients are at high risk of life-threatening complications such as aortic rupture, stroke, visceral ischemia, cardiac tamponade, and circulatory failure. The aim of surgery in proximal type A (type I, II) aortic dissection is prevention of rupture or development of pericardial effusion which may lead to cardiac tamponade and death. Similarly, sudden onset of aortic regurgitation and coronary flow obstruction requires urgent surgical attention with the aim to resect the region of intimal tear in dissection limited to the ascending aorta and replacement by a composite or interposition graft (if the aortic valves are intact or resuspendable). When the dissection extends to the aortic arch or the descending aorta, resection of the entire intimal flap may not be possible or the patient may require partial or total arch replacement.61 A recent report highlights the problem of either resecting or leaving unrecognized intimal tears in the arch or descending thoracic aorta, which is seen in 20%-30% and predisposes to later distal aortic reoperation.62 Considering an operative mortality between 15% and 35% even in centers of excellence, adjunctive measures such as profound hypothermic circulatory arrest and selective retrograde perfusion of head vessels have been used in the surgical management of arch repair or an open distal anastomosis.63 Whereas selective head perfusion recently gained acceptance for improved outcome with a 5-year survival of 73% (6)%, profound hypothermic circulatory arrest failed to improve early complications, survival and distal reoperation rates in patients with acute type A dissection; 30-day, 1-year and 5-year survival estimates were 81% (2)%, 74% (3)% and 63% (3)%, and thus not different from other techniques using propensity-matched retrospective analysis.64 The key to success is rapid surgery prior to any hemodynamic instability or deterioration (Table 4).
Once the patient is on extracorporal circulation and preferably antegrade cerebral perfusion, which is usually established after cannulation of one femoral artery and the right atrium, the aorta is mobilized to visualize the innominate artery and the aortic root. If the valve leaflets are intact, aortic valve reconstruction using David's or Yacoub's resuspension technique is gaining growing acceptance over valve replacement.65,66
The approach to an acute type A (type I, II) dissection (Figure 1) in an ectatic proximal aorta requires a different approach. In such instances, mostly in patients with the Marfan's syndrome, a composite graft (aortic tube graft with integrated valve) is preferred with coronary re-implantation.67-69 Valve sparing operations are delicate endevours in an emergency and require great surgical competence in centers with expertise in elective cases. If the dissection compromises the left or right ostium without disrupting the coronary vessel, the ostium can usually be preserved. An ostium completely surrounded by dissected aortic wall may be excised in button form. The dissected layers around the ostium are conjoined using tissue adhesive and over and over suturing before the anastomosis to a tube graft is accomplished. Bypass grafting of coronary arteries using saphenous vein segments is limited to those instances where a small torn ostium precludes reconstruction. Although definitive treatment of acute type A aortic dissections includes surgery, about 20% of patients are not operated. The reasons cited for medical therapy were comorbid conditions, old age (mean, 80 years), and patient refusal. Best treatment of the acutely dissected aortic arch remains an unresolved issue. At present there is growing consensus that any dissected arch should be explored during hypothermic circulatory arrest. In the absence of an arch tear, an open distal anastomosis of the graft and the conjoined aortic wall layers at the junction of the ascending and arch portions is justified. Arch tears occur in up to 30% of patients with acute dissection.70,71 Whenever extensive tears are found, which continue beyond the junction of the transverse and descending aortic segments, or with an acute dissection of a previously aneurysmatic arch, subtotal or total arch replacement may be required with reconnection of some or all supraaortic vessels to the graft during hypothermic circulatory arrest and antegrade head perfusion.72
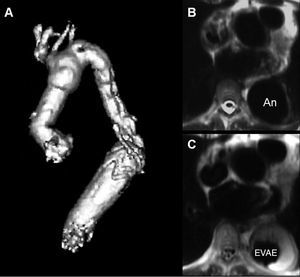
In dissecting and non-dissecting aneurysms extending to the downstream aorta an elephant trunk extension of the arch graft is an option described by Borst et al.73 This technique greatly facilitates later procedures on the downstream aorta. Instead of performing a conventional anastomosis between the end of the graft and the descending aorta, the graft is allowed to float freely in the aortic lumen. In a later procedure the elephant trunk section of the graft may either be connected surgically to the distal descending aorta directly or extended with another tubular prosthesis, or interventionally by a customized endovascular stent-graft which may then be anastomosed at any desired downstream level of the aorta (Figure 5).
Figure 5. A. Reconstructed MRI after percutaneous use of a customized stent-graft (A) to connect a surgically inserted elephant trunk with the upper abdominal aorta in order to exclude an aneurysm that had formed at the distal end of the elephant trunk (B) after placement of the customized stent-graft, the thoracic aneurysm was successfully excluded from circulation with thrombus formation around the stent-graft protheses (C).
Role of Endovascular Procedures in Type A (Proximal) Dissection
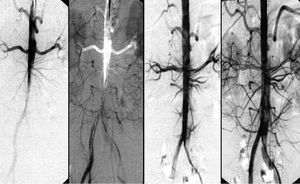
Conventional treatment of Stanford type A (de Bakey type I, II) dissection consists of surgical reconstruction of the ascending aorta with complete or partial resection of the dissected aortic segment; endovascular strategies have no clinical application except to relieve critical malperfusion prior to surgery of the ascending aorta by stent-graft placement in the thoracic descending aorta or by distal fenestration in rare cases of thoracoabdominal extension (de Bakey type I) with peripheral ischemic complications. Reconstruction of a collapsed true lumen might result in reestablishment of sidebranch flow (Figure 6) and re-established distal run-off.74-77 Most scenarios of malperfusion syndrome are amenable to endovascular management considering that surgical mortality rates in patients with acute peripheral vascular ischemic complications are similar to those with mesenteric ischemia, and reach 89% in-hospital mortality.78,79
Figure 6. Malperfusion of distal aorta by occlusive type B dissection. Stent-graft placement in the true lumen of the proximal descending aorta re-established flow to the abdomen and legs.
Descending (Type B) Aortic Dissection
In the current era, endovascular interventions for acute descending (type B) aortic dissection is the primary approach in the setting of complications of acute distal dissection because surgical repair has no proven superiority over medical or interventional treatment in this scenario. Patients with uncomplicated aortic dissections confined to the descending thoracic aorta (Stanford type B or deBakey type III) are at present treated with medical therapy, but may be considered candidates for endovascular therapy in selected cases in the near future. Medical treatment focuses on invasive hemodynamic monitoring, β-blockade, and vasodilators if needed to keep systolic blood pressure ¾120 mm Hg. Pain control with morphine sulfate is also important to attenuate the sympathetic release of catecholamines to pain with resultant tachycardia and hypertension. Once the patient is stable, oral β-blockers and other antihypertensive medications are continued under close follow-up with imaging and clinical assessment at six-month intervals. In a series of 384 patients with type B dissections from IRAD, 73% were managed medically. In-hospital mortality for these patients was 10%26 The reported long-term survival rate with medical therapy is 60% to 80% at 4 to 5 years and 40% to 45% at 10 years.80-82 Survival is best in patients with noncommunicating dissections, or fully thrombosed false lumen.
Surgery in Type B (Distal) Aortic Dissection
In the current eracute type B (type III) are limited to the prevention or relief of life-threatening complications such as intractable pain, rapidly expanding aortic diameter, or signs of imminent aortic rupture, but can also be managed by interventional stent-graft placement. The onset of complications such as malperfusion of vital aortic sidebranches warrants interventional therapy by stent-grafting to improve distal true lumen flow or in rare instances catheter-guided fenestration of an occlusive lamella. When this approach does not lead to prompt relief of symptoms, surgical intervention may still be required. At present uncomplicated type B (type III) aortic dissections are usually treated conservatively, since surgical repair has no proven superiority over medical or interventional treatment in stable patients. In complicated cases the concept of interventional stent-graft placement is currently being explored.4-2021-5051-76 Furthermore, because patients treated surgically are primarily those with a complicated course, the short-term mortality for such patients is higher than with medical therapy.26
Interventional Endovascular Stent-Grafting in Type B Dissection
The concept of emergent stent-graft placement for urgent endovascular aortic repair of dissection is attractive, and a growing number of acute type B aortic dissection is subjected to endovascular repair with little evidence of periprocedural morbidity leading to aborted malperfusion or sealed leakage, and eventually to reconstruction of the dissected aorta; stent-graft placement in complicated distal aortic dissection is an emerging concept associated with few peripheral or neurological complications in experienced hands83-85 and with better short- and midterm outcome than surgical or medical treatment in high risk groups of type B dissection.
The exact role of percutaneous fenestration and stent placement in aortic dissection is still evolving even in patients with life threatening complications manifested by end organ ischemia. The mortality rate of patients with renal ischemia is 50% to 70% and as high as 87% in mesenteric ischemia. Considering a surgical in-hospital mortality rate in the setting of end organ ischemia as high as 89%, percutaneous management of this complication has emerged as a viable option before or after definitive surgical management.
Ten years ago Nienaber et al compared the outcome of stent grafting with open surgery in a nonrandomized evaluation of 24 patients with chronic type B aortic dissection with at least one indication for surgery.75 Stent graft placement resulted in no morbidity or mortality, whereas surgery for type B dissection was associated with 4 deaths (33%) and 5 serious adverse events within 12 months. Dake et al studied the placement of endovascular stent grafts across the primary entry tear in 19 patients with acute aortic dissection (4 patients with type A and 15 with type B).76 Dissections involved aortic branches in 14 of the 19 patients (74%) and symptomatic compromise of multiple branch vessels was observed in 7 patients (37%). Placement of stent graft across the primary tear was technically successful in all 19 patients. Complete thrombosis of the false lumen was achieved in 15 patients (79%). Revascularization of ischemic branch vessel was successful in 76% of the obstructed branches. 3 of 19 (16%) patients died at 30 days without further deaths during the subsequent average follow up of 13 months.
The European Society of Cardiology Task force on acute aortic dissection released it's recommendations for the indications for stent graft and/or fenestration.87 Additionally, in high risk patients not suitable for surgery because of age, comorbid conditions, or personal preference, endovascular repair offers palliative treatment to those who otherwise would have been left to follow the natural history of the disease.
From the IRAD registry we learnt that 73% of type B dissection cases were managed medically; in-hospital mortality for these patients was 10 percent.86 The reported long-term survival rate with medical therapy is approximately 60%-80% at 4-5 years and approximately 40%-45% at 10 years.88-90 Survival is best in patients with noncommunicating and retrograde dissections.
In the current era, endovascular stent-graft intervention for acute descending (Type B) aortic dissection is reserved for complications of the disease considering that surgical repair has no proven superiority over medical or interventional treatment in stable patients. Patients with uncomplicated aortic dissections confined to the descending thoracic aorta (Stanford type B or deBakey type III) are best treated with medical therapy. Medical treatment consists of invasive hemodynamic monitoring, beta blockade, and arterial vasodilators if needed to keep systolic blood pressure less than 120 mm Hg. Pain control with morphine sulfate is also important to attenuate the sympathetic release of catecholamines to pain with resultant tachycardia and hypertension. Once the patient is stable, oral beta-blockers and other antihypertensive medications if necessary are substituted and the patient is discharged with very close follow up.
There is consensus that indications for endovascular procedure in the setting of acute type B aortic dissections are limited to prevention or relief of life-threatening complications. Similar to open surgery these complications include aortic rupture, ischemia of limbs and organ systems, renal hypertension, persistent or recurrent intractable pain, progression of dissection and aneurysm expansion, all more likely with a patent false lumen and enlarging aortic diameter (Figure 7) and uncontrolled hypertension. In most series, classic open operations for acute type B aortic dissections carry a higher mortality that historically ranges between 35%-75%. Furthermore, because patients with a complicated course may preferentially undergo endovascular procedures than surgery which may lower the short term mortality for such patients (Figure 8).86
Figure 7. Actuarial survival curves for patients classified by thrombosis of the false lumen and total aortic diameter. (Taken from Ref. 106.)
Figure 8. Survival curves due to acute type B aortic disse ction for all patients and by management group based on Kaplan-Meier analysis of 40-day mortality (PI=percutaneous intervention). (Taken from Ref. 86.)
Current Therapeutic Strategies
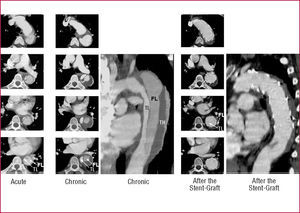
Recent reports support the notion that percutaneous stent-graft placement in the dissected aorta is safer and produces better results than surgery for type B dissection.91 Results of short-term follow-up are excellent with a 1-year survival rate of >90%; tears can be readapted and aortic diameters generally decrease with complete thrombosis of the false lumen. This suggests that stent placement may facilitate healing of the dissection, sometimes of the entire aorta, including abdominal segments (Figure 9).
Figure 9. Type B aortic dissection in a 48-year old man; note the dynamic obstruction of the true lumen (TL) in the acute phase. After stent-graft placement across the proximal thoracic entry, the entire true lumen of the thoracic aorta is reconstructed with time, with complete "healing" of the dissected aortic wall and shrinking of the completely thrombosed false lumen (FL). TH indicates thrombus.
The EUROSTAR/United Kingdom registry report represents a first large series of patients treated with thoracic aortic stent-grafts; in this combined registry, 131 patients with aortic dissection (5% proximal, 81% distal, 14% not classified) were treated with stent-grafts, with 57% presenting symptoms of rupture, aortic expansion, or side branch occlusion. Although meaningful long-term data still lacking, primary technical success was achieved in 89%, at the expense of a 30-day mortality of 8.4%.25 Similar to the metaanalysis91 paraplegia occurred in 0.8% of those treated, and survival at 1 year after treatment was reported in 90%.92
A series of patients at the Arizona Heart Institute, comprising 40 patients with a thoracic endograft for complicated distal aortic dissection, enjoyed a technical success in 95%. There was one perioperative death due to iliac rupture and one case of paraplegia, while 15 patients (38%) experienced transient post-operative complications, mostly renal and pulmonary; 1-year survival was 85%. Of the patients available for follow up CT scan, 97% (30 of 31 patients) exhibited a stable or decreasing aortic diameter and no rupture occurred during the study period, justifying the conclusion that thoracic aortic stent grafting stabilized the aorta and decreased incidence of late expansion and aortic rupture.93
In a series of 24 cases of high-risk distal aortic dissection (16 acute, 8 chronic), peri-procedural mortality was 13% due to rupture with associated endoleak in one and retrograde dissection with cardiac tamponade in two; one patient survived open conversion of retrograde dissection. The three cases of retrograde dissection were all associated with an open wire stent graft design deployed within the thoracic arch. Overall, 2-year mortality was 17% in this emergent and high-risk patient population.94 Similarly, of 43 patients with complicated distal aortic dissection (24 acute, 19 chronic) 3 cases developed abrupt retrograde dissection following stent graft deployment (open stent design) leading to cardiac tamponade and death; 2 patients died during follow up from progressive dissection despite stent-grafting of the entry tear. In the remaining 38 patients (mean follow up of 20 months), all survived and developed thrombosis of the false lumen.95 The Talent Thoracic Retrospective (TTR) registry is at present the largest study on long term results in a wide cohort of patients (457), and more than 3 years clinical and imaging follow-up in 95 patients; early results confirm findings of smaller series with an in-hospital mortality was 5%, including 113 patients treated under emergency condition.96 An advantage of endovascular repair in acute aortic syndrome was seen in reduced blood loss97,98 compared to open surgery, in which back-bleeding from branch arteries and anastomotic sites or from iatrogenic venous injuries could precipitate shock and neurological sequelae. Most peri-operative complications in emergency endovascular cases of this study arose from preexisting medical conditions and were not procedure-related. Moreover, in patients with traumatic aortic rupture (85 patients with no mortality), endovascular stent-graft treatment strongly abated the high mortality of conventional open surgical repair.99 In patients with multiple trauma, the option of nonsurgical treatment of the aortic lesion may allow prompt management of trauma-associated lesions, and improved overall survival. The feasibility of endovascular treatment in patients with Marfan syndrome is controversial, because of the particular fragility of the aortic wall but outcomes after stent-grafts may be acceptable.96 At present, the most frequent complications in endovascular procedure are stroke (3.7%) and vascular lesions at the access site (3.2%), both probably related to the difficult advancement and manipulation of the large bore (22-27F) and inflexible delivery system. The only significant predictor of stroke was overstenting of the left subclavian artery without previous revascularization although a recent report99 suggests relative safety if some precautions are met.100. Nevertheless, endovascular stent-graft has potential for reducing the risk for paraplegia by avoiding crossclamping, significant blood loss and severe hypotension. Yet, concomitant or previous abdominal aneurysm repair has been reported as a risk factor for spinal ischemia.101,102 Long-segment thoracic aortic exclusion was the most important predictor of spinal ischemia, regardless of dissection or aneurysm. Considering the possibility of late complications, surveillance imaging using MR or CT should be offered after 3 and 12 months followed by further examinations in yearly intervals. In some patients, follow-up imaging has revealed tears that had initially been overlooked, but required additional stents.
The INvestigation of STEnt grafts in patients with type B Aortic Dissection (INSTEAD) trial, is the first randomized trial investigating the role of stent-graft treatment of uncomplicated type B aortic dissection compared with best medical therapy alone.103 Thus, the INSTEAD trial will address whether patients with chronic, uncomplicated, distal aortic dissection treated with an endovascular stent-graft have an improved initial outcome and freedom from late dissection complications. At present, no thoracic endograft device is specifically approved for the treatment of aortic dissection; however, such devices are being used off label for this purpose, and there are plans for upcoming trials for endovascular stent-graft treatment of complicated and non-complicated aortic pathologies.
Although preliminary data suggest that stent-graft repair may ultimately become the treatment of choice for most patients with distal aortic syndromes, the available evidence to date does not justify indiscriminate use of this technology in patients currently managed with medical therapy alone. Comparative clinical trials are clearly needed to clarify the role of stent-graft repair in acute aortic syndromes. Even if patients at high surgical risk will benefit from endovascular technology, the exact role of stent grafting remains to be defined as we continue to accumulate long-term data and experience and as devices and techniques evolve.
Intramural Hematoma
Similar to type A and B aortic dissection, surgery is advocated in patients with type A IMH and initial medical therapy in patients with type B IMH. A meta-analysis of 143 patients found that patients with lesions of the ascending aorta had a lower mortality with surgery than medical treatment (14% vs 36%). Patients with lesions of the descending aorta had a similar mortality with medical or surgical therapy (14% vs 20%).36 The cardiology and surgical community has generally concluded that acute IMH involving the ascending aorta should be managed surgically because of an unacceptably high mortality with medical treatment.17-21,31,104,105 Given these uncertainties, many experts recommend aortic repair for acute IMH of the ascending aorta similar to type A dissection and aggressive medical therapy for IMH in the descending aorta similar to type B dissection.105
CONCLUSIONS
Much has been learned about the risk factors, clinical characteristics, diagnosis, and management of acute aortic syndrome over the last decade. Technological advances in imaging techniques and a better understanding of the pathobiology of acute aortic dissection have led to the discovery of variants of aortic pathologies now called acute aortic syndromes. Furthermore, diverse surgical and percutaneous strategies to treat aortic syndromes are continuing to improve and evolve. As a result of knowledge and interest in this area, the outcomes of patients treated for acute aortic syndromes have improved. However, there is still much work to be done. The use of diagnostic and therapeutic pathways that facilitate efficient and streamlined care similar to acute coronary syndromes or stroke has the potential to improve patient outcomes. Finally, with continued enthusiasm in learning more about this disorder in concert with continued improvements in the diagnosis and management of this disease, further advances are on the horizon.
This section is sponsored by Laboratorio Dr. Esteve
Correspondence: Dr. Christoph A. Nienaber,
Division of Cardiology, University Hospital Rostock,
Rostock School of Medicine,
Ernst-Heydemann-Str., 6, 18057 Rostock, Germany,
E-mail: christoph.nienaber@med.uni-rostock.de