Mortality from left-sided infective endocarditis remains very high. The aim of this study was to assess the impact of a multidisciplinary alert strategy (AMULTEI), based on clinical, echocardiographic and microbiological findings, implemented in 2008 in a tertiary hospital.
MethodsCohort study comparing our historical data series (1996-2007) with the number of patients diagnosed with left-sided endocarditis from 2008-2011 (AMULTEI).
ResultsThe AMULTEI cohort included 72 patients who were compared with 155 patients in the historical cohort. AMULTEI patients were significantly older (62.5 vs 57.9 years in the historical cohort; P=.047) and had higher comorbidity (Charlson index, 3.33 vs 2.58 in the historical cohort; P=.023). There was also a trend toward more enterococcal etiology in the AMULTEI group (20.8% vs 11.6% in the historical cohort; P=.067). In the AMULTEI group, early surgery was more frequently performed (48.6% vs 23.2%; P<.001) during hospitalization, the incidence of septic shock was significantly lower (9.7% vs 24.5%; P=.009) and there was a trend toward reductions in neurological complications (19.4% vs 29.0%; P=.25) and severe heart failure (12.5% vs 18.7%; P=.24). In-hospital mortality and mortality during the first month of follow-up were significantly lower in the AMULTEI group (16.7% vs 36.1%; P=.003).
ConclusionsDespite the trend toward older age and more comorbidity measured by the Charlson index, early mortality was significantly lower in patients treated with the AMULTEI strategy.
Keywords
Left-sided infective endocarditis (LSIE) leads to very high short-term mortality. Despite advances in diagnosis and treatment, LSIE mortality is still 15% to 25% for native valves and 30% to 50% for prosthetic valves.1–3 The persistently high mortality could be explained by the presence of more virulent organisms and an aging population with greater comorbidity.4–6 In recent years, the frequency of staphylococcal etiology has increased, and there are increasing reports of infections in prosthetic valves, intracardiac devices and, lately, stents. In addition, the vancomycin minimum inhibitory concentration has increased in some strains of methicillin-resistant Staphylococcus aureus and coagulase-negative Staphylococcus.7
Because intracardiac complications are often severe and fatal, it has been proposed that prognosis could be improved by an early more aggressive treatment with early surgical indications,8,9 especially if this treatment is accompanied by the early administration of effective antibiotic therapy to reduce the frequency of cardiac embolism or septic shock, both of which are strongly associated with mortality.10
An analysis performed at our center in 2007 indicated an overall early mortality of 36%. A hypothesis was then proposed that this mortality could be reduced by shortening the time to diagnosis and performing early medical and surgical treatment. To this end, the AMULTEI (Alerta Multidisciplinaria en Endocarditis Infecciosa [Multidisciplinary Alert in Infective Endocarditis]) strategy was proposed, which is a new form of multidisciplinary organization for the care of patients with LSIE in our hospital.
The aim of this study was to analyze the effect of the AMULTEI strategy on early mortality in patients with LSIE.
METHODSThis prospective cohort study included patients consecutively diagnosed with LSIE using the modified Duke criteria from 2008 to 2011 (inclusive) and treated with the AMULTEI multidisciplinary care strategy. This series was compared with a historical cohort of patients diagnosed with left-sided endocarditis at our center from 1996 to 2007, whose information was collected prospectively beginning in 1996.
The study was performed in a tertiary hospital with 717 beds; the hospital has a cardiac surgery service and is a referral center for this condition. This hospital works in collaboration with other hospitals in Andalusia within the Andalusian Group for the Study of Cardiovascular Infections of the Andalusian Infectious Disease Society and since 2008 has collaborated with other hospitals nationwide within the Spanish Group for the Management of Endocarditis in Spain and the Spanish Society for the Study of Cardiovascular Infections. This hospital also collaborates with the Spanish Network for Research on Infectious Diseases as an associate clinical group in the “Infective Endocarditis Health Problem” and in the Thematic Network of Cooperative Research on Cardiovascular Diseases.
The AMULTEI StrategyThe multidisciplinary alert for endocarditis has 3 key domains: clinical (mainly internal medicine, infectious diseases), microbiological, and echocardiographic. An attending physician was appointed in each of these areas. These physicians communicate by telephone if there is a clinical, echocardiographic or microbiological finding compatible with infective endocarditis and facilitate a comprehensive patient assessment as a priority measure. If there is a positive diagnosis (modified Duke criteria11), the cardiac surgery service is involved and remains aware of the patient's progress, regardless of whether there is an initial surgical indication.
Clinical StudyThe primary dependent variable of the study, early mortality, was defined as mortality occurring in-hospital or during the first month after discharge. Prosthetic endocarditis was considered to be at an early stage when diagnosed within the first year of implantation and at a late stage when diagnosed after that. Patients with suspected infective endocarditis were assessed before admission to the emergency room by the on-call internal medicine and cardiology teams, who started empirical antibiotic therapy after blood sample extraction for culture.
The Charlson comorbidity index12 was assessed at admission in all patients. A Charlson index score≥3 was considered high. Heart failure was diagnosed according to the Framingham criteria and was considered severe when intravenous inotropic therapy or mechanical ventilation was required. Septic shock was defined according to routine standards.13 Acute renal failure was diagnosed as serum creatinine>1.5mg/dL in patients with normal baseline renal function or a decline>25% in creatinine clearance in patients with chronic renal failure. Elderly patients were considered those aged 75 years or older.
Central nervous system disease was considered in cases of meningitis, brain embolism, or hemorrhage and encephalopathy, with confirmatory findings in radiological images, cerebrospinal fluid analysis or autopsy.
The potential surgical risk was estimated by EuroSCORE I. Cardiac surgery was considered to be delayed when the patient underwent the intervention after more than 48h in severe heart failure or after 7 days with progressive heart failure despite medical treatment. Time to surgery was measured from the date of hospital admission to the date of surgery.
Microbiological StudyThe microbiological study was performed using blood cultures (BACTEC® automated system), a valve culture, or a culture of another specimen obtained during surgery. In the AMULTEI cohort, pan-bacterial C-reactive protein was also analyzed in surgical specimens. Blood cultures were obtained by direct venipuncture, with follow-up tests 1 week after stopping treatment and at 2 months after discharge.
Echocardiographic StudyTransthoracic echocardiography was requested for all patients with clinical or microbiological suspicion of endocarditis. Transesophageal echocardiography was performed in all patients who had a negative or inconclusive transthoracic study or who had prosthetic valves, following the strategy recommended in the literature,14 and subsequently ratified by the committee of experts on endocarditis of the European Society of Echocardiography.15 Monoplane or biplane transesophageal probes were used until 2008 and multiplane probes thereafter. The study was performed in the echocardiography laboratory or in the intensive care unit if the patient was hemodynamically unstable or required mechanical ventilation. Furthermore, since 2008, intraoperative transesophageal echocardiography was performed in all patients. A vegetation was diagnosed based on images of a movable mass located in valvular structures or the impact zone of pathological jets with vibration or erratic movement different from the valve structure where they were located. Its size and degree of mobility was then confirmed. An abscess was defined as the presence of hypoechoic areas or irregular echogenicity without evidence of interior flow, situated in the vicinity of the affected valve. Pseudoaneurysms were diagnosed by the presence of anechoic areas with interior flow and systolic expansion. Finally, fistulas were defined as abnormal communications between 2 heart chambers. The degree of valvular dysfunction was quantified following the American Society of Echocardiography guidelines.16
Statistical AnalysisSPSS 15.0 software for Windows was used for the statistical analysis.
Quantitative variables were expressed as means (standard deviation). Comparisons of means between groups were performed using Student's t test, and the 95% confidence interval (95%CI) was obtained for the mean difference.
Qualitative variables were expressed as percentages; their association was established by odds ratios (OR) and their 95%CI, and statistical significance, by the χ2 test.
Multivariate analysis was performed using logistic regression. For this analysis, all variables associated with mortality in the univariate analysis were included, as well as those traditionally associated with mortality, following a review of the scientific literature. The alpha error was 5%.
To study mortality, Kaplan-Meier curves were also constructed.
RESULTSA total of 227 LSIE patients diagnosed according to the Duke criteria were studied; 72 (31.7%) were treated with the AMULTEI strategy and 155 (68.3%) belonged to the historical cohort (1996-2007).
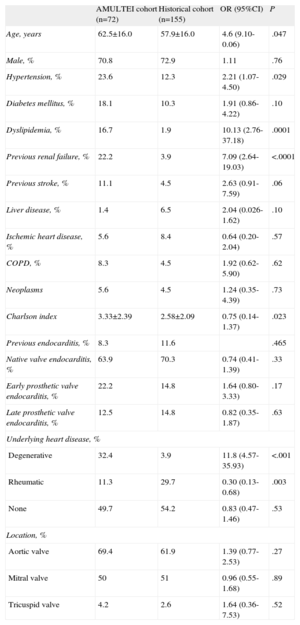
The clinical features of both groups of patients are compared in Table 1. Patients treated with the AMULTEI strategy were significantly older (62.5 [16.0] vs 57.9 [16.0] years; P=.047) and had more comorbidity at diagnosis with a significantly higher Charlson comorbidity index score (3.33 [2.39] vs 2.58 [2.09]; P=.023). There were no differences in sex.
General Characteristics of the Study Population
| AMULTEI cohort (n=72) | Historical cohort (n=155) | OR (95%CI) | P | |
| Age, years | 62.5±16.0 | 57.9±16.0 | 4.6 (9.10-0.06) | .047 |
| Male, % | 70.8 | 72.9 | 1.11 | .76 |
| Hypertension, % | 23.6 | 12.3 | 2.21 (1.07- 4.50) | .029 |
| Diabetes mellitus, % | 18.1 | 10.3 | 1.91 (0.86-4.22) | .10 |
| Dyslipidemia, % | 16.7 | 1.9 | 10.13 (2.76-37.18) | .0001 |
| Previous renal failure, % | 22.2 | 3.9 | 7.09 (2.64-19.03) | <.0001 |
| Previous stroke, % | 11.1 | 4.5 | 2.63 (0.91-7.59) | .06 |
| Liver disease, % | 1.4 | 6.5 | 2.04 (0.026-1.62) | .10 |
| Ischemic heart disease, % | 5.6 | 8.4 | 0.64 (0.20-2.04) | .57 |
| COPD, % | 8.3 | 4.5 | 1.92 (0.62-5.90) | .62 |
| Neoplasms | 5.6 | 4.5 | 1.24 (0.35-4.39) | .73 |
| Charlson index | 3.33±2.39 | 2.58±2.09 | 0.75 (0.14-1.37) | .023 |
| Previous endocarditis, % | 8.3 | 11.6 | .465 | |
| Native valve endocarditis, % | 63.9 | 70.3 | 0.74 (0.41-1.39) | .33 |
| Early prosthetic valve endocarditis, % | 22.2 | 14.8 | 1.64 (0.80-3.33) | .17 |
| Late prosthetic valve endocarditis, % | 12.5 | 14.8 | 0.82 (0.35-1.87) | .63 |
| Underlying heart disease, % | ||||
| Degenerative | 32.4 | 3.9 | 11.8 (4.57-35.93) | <.001 |
| Rheumatic | 11.3 | 29.7 | 0.30 (0.13-0.68) | .003 |
| None | 49.7 | 54.2 | 0.83 (0.47-1.46) | .53 |
| Location, % | ||||
| Aortic valve | 69.4 | 61.9 | 1.39 (0.77-2.53) | .27 |
| Mitral valve | 50 | 51 | 0.96 (0.55-1.68) | .89 |
| Tricuspid valve | 4.2 | 2.6 | 1.64 (0.36-7.53) | .52 |
95%CI, 95% confidence interval; AMULTEI, Alerta Multidisciplinaria en Endocarditis Infecciosa (Multidisciplinary Alert in Infective Endocarditis); COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Categorical variables are expressed as percentages and odds ratios; continuous variables as mean±standard deviation, mean difference and 95% confidence interval of the mean difference.
No significant differences were identified according to whether native or prosthetic valves were involved, although there was a higher frequency of early prosthetic valve endocarditis and of LSIE with concomitant involvement of intracardiac devices in the AMULTEI cohort (Table 1).
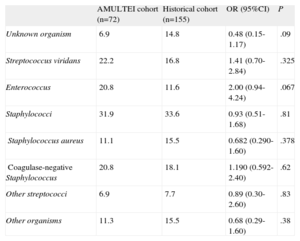
The spectrum of microorganisms causing the endocarditis differeded between the groups (Table 2), but did not reach statistical significance. The AMULTEI cohort exhibited a trend toward a lower percentage of cases caused by an unknown organism (6.9% vs 14.8%; P=.09) and a higher percentage of enterococcal endocarditis (20.8% vs 11.6%; P=.067).
Spectrum of Organisms Causing Endocarditis
| AMULTEI cohort (n=72) | Historical cohort (n=155) | OR (95%CI) | P | |
| Unknown organism | 6.9 | 14.8 | 0.48 (0.15-1.17) | .09 |
| Streptococcus viridans | 22.2 | 16.8 | 1.41 (0.70-2.84) | .325 |
| Enterococcus | 20.8 | 11.6 | 2.00 (0.94-4.24) | .067 |
| Staphylococci | 31.9 | 33.6 | 0.93 (0.51-1.68) | .81 |
| Staphylococcus aureus | 11.1 | 15.5 | 0.682 (0.290-1.60) | .378 |
| Coagulase-negative Staphylococcus | 20.8 | 18.1 | 1.190 (0.592-2.40) | .62 |
| Other streptococci | 6.9 | 7.7 | 0.89 (0.30-2.60) | .83 |
| Other organisms | 11.3 | 15.5 | 0.68 (0.29-1.60) | .38 |
95%CI, 95% confidence interval; AMULTEI, Alerta Multidisciplinaria en Endocarditis Infecciosa (Multidisciplinary Alert in Infective Endocarditis); OR, odds ratio.
Transthoracic echocardiography was performed in 100% of AMULTEI patients vs 98.7% of the historical cohort. A transesophageal study was further undertaken in 73.6% of AMULTEI patients vs 60.8% in the historical cohort (P=.06). The echocardiogram showed vegetations in 66.7% of AMULTEI patients vs 65.8% of the historical cohort (P=.89) and perivalvular involvement in 22.2% vs 16.8% (OR=1.41; 95%CI, 0.70-2.85; P=.33), respectively. Of the patients with no vegetations detected by echocardiography, 29.2% of the AMULTEI cohort and 32.1% of the historical cohort had perivalvular involvement. Paravalvular leak was diagnosed in 16.7% and 15.5%, respectively.
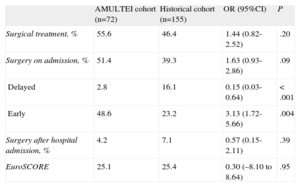
Cardiac surgery was performed during hospitalization in 51.4% of AMULTEI patients vs 39.3% of the historical cohort (P=.09) (Table 3). Delayed cardiac surgery was performed after discharge in 4.2% vs 7.1% (P=.39). Early surgery was significantly more frequent in the AMULTEI cohort (48.6% vs 23.2%; P<.001). Simultaneously, the percentage of delayed surgery was significantly lower (Table 3). The time from admission to surgery was 14.5 (20.9) days for AMULTEI patients and 15.1 (21.0) days for the historical cohort (P=.85). The mean hospital stay was 37.4 days compared with 36.4 days.
Surgical Treatment in Each Cohort
| AMULTEI cohort (n=72) | Historical cohort (n=155) | OR (95%CI) | P | |
| Surgical treatment, % | 55.6 | 46.4 | 1.44 (0.82-2.52) | .20 |
| Surgery on admission, % | 51.4 | 39.3 | 1.63 (0.93-2.86) | .09 |
| Delayed | 2.8 | 16.1 | 0.15 (0.03-0.64) | < .001 |
| Early | 48.6 | 23.2 | 3.13 (1.72-5.66) | .004 |
| Surgery after hospital admission, % | 4.2 | 7.1 | 0.57 (0.15-2.11) | .39 |
| EuroSCORE | 25.1 | 25.4 | 0.30 (–8.10 to 8.64) | .95 |
95%CI, 95% confidence interval; AMULTEI, Alerta Multidisciplinaria en Endocarditis Infecciosa (Multidisciplinary Alert in Infective Endocarditis); OR, odds ratio.
The overall operative mortality was reduced from 37.7% in the historical cohort to 13.5% in the AMULTEI cohort (OR=0.25; 95%CI, 0.09-0.75; P=.01). Mortality in patients undergoing early surgery was also reduced, being 33.3% in the historical cohort and 14.3% in the AMULTEI cohort (OR=0.63; 95%CI, 0.41-0.96; P=.06). Mortality in patients undergoing delayed surgery in the historical cohort was 44%. Delayed surgery was performed in only 2 patients in the AMULTEI cohort, and both survived.
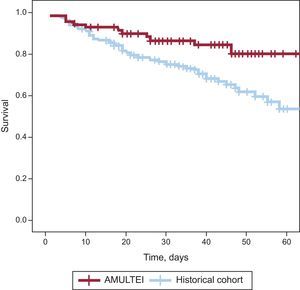
In-hospital mortality and mortality during the first month after hospital discharge was 16.7% in the AMULTEI cohort compared with 36.1% in the historical cohort (OR=0.35; 95%CI, 0.17-71.30; P=.003). The figure shows the Kaplan Meier survival curves for each cohort.
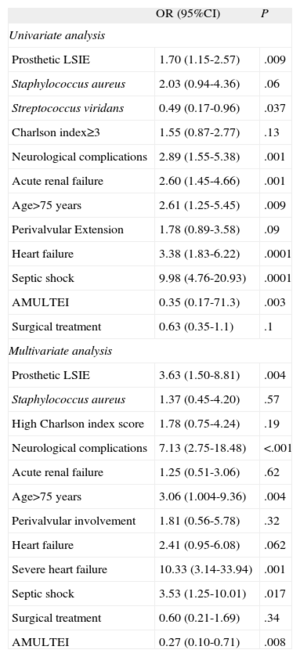
The primary factors associated with hospital mortality in the univariate analysis are listed in Table 4. The strongest statistical associations were found with the onset of septic shock, acute heart failure or kidney failure, advanced age, the presence of neurological symptoms, and the presence of a prosthetic LSIE. Isolation of Streptococcus viridans or the AMULTEI strategy showed a protective role. Performing cardiac surgery was associated with a nonsignificant trend toward reduced mortality.
Factors Associated With Early Mortality
| OR (95%CI) | P | |
| Univariate analysis | ||
| Prosthetic LSIE | 1.70 (1.15-2.57) | .009 |
| Staphylococcus aureus | 2.03 (0.94-4.36) | .06 |
| Streptococcus viridans | 0.49 (0.17-0.96) | .037 |
| Charlson index≥3 | 1.55 (0.87-2.77) | .13 |
| Neurological complications | 2.89 (1.55-5.38) | .001 |
| Acute renal failure | 2.60 (1.45-4.66) | .001 |
| Age>75 years | 2.61 (1.25-5.45) | .009 |
| Perivalvular Extension | 1.78 (0.89-3.58) | .09 |
| Heart failure | 3.38 (1.83-6.22) | .0001 |
| Septic shock | 9.98 (4.76-20.93) | .0001 |
| AMULTEI | 0.35 (0.17-71.3) | .003 |
| Surgical treatment | 0.63 (0.35-1.1) | .1 |
| Multivariate analysis | ||
| Prosthetic LSIE | 3.63 (1.50-8.81) | .004 |
| Staphylococcus aureus | 1.37 (0.45-4.20) | .57 |
| High Charlson index score | 1.78 (0.75-4.24) | .19 |
| Neurological complications | 7.13 (2.75-18.48) | <.001 |
| Acute renal failure | 1.25 (0.51-3.06) | .62 |
| Age>75 years | 3.06 (1.004-9.36) | .004 |
| Perivalvular involvement | 1.81 (0.56-5.78) | .32 |
| Heart failure | 2.41 (0.95-6.08) | .062 |
| Severe heart failure | 10.33 (3.14-33.94) | .001 |
| Septic shock | 3.53 (1.25-10.01) | .017 |
| Surgical treatment | 0.60 (0.21-1.69) | .34 |
| AMULTEI | 0.27 (0.10-0.71) | .008 |
95%CI, 95% confidence interval; AMULTEI, Alerta Multidisciplinaria en Endocarditis Infecciosa (Multidisciplinary Alert in Infective Endocarditis); LSIE, left-sided infective endocarditis; OR, odds ratio.
The following factors were significantly associated with mortality in the multivariate analysis: the AMULTEI strategy regarding its protective role and the development of severe heart failure, septic shock, and prosthetic valve endocarditis or neurological manifestations, and advanced age as independent predictors of mortality.
A multivariate model was also constructed that incorporated the historical cohort, further dividing it into 3 separate, 4-year periods, along with the AMULTEI cohort; these 3 historical periods were identified as independent predictors of mortality very close to significance: period 1, OR=5.06 (95%CI, 1.60-15.90; P=.006), period 2, OR=2.90 (95%CI, 0.93-9.01; P=.065), and period 3, OR=2.70 (95%CI, 0.96-7.42; P=.059).
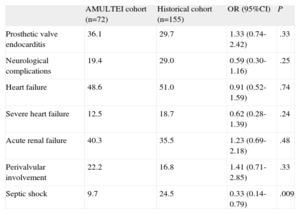
The distribution of all of the factors associated with mortality in each cohort is shown in Table 5. There was a trend toward a decrease in the incidence of severe heart failure and neurological symptoms, and a significant reduction in cases of septic shock, in the AMULTEI cohort.
Distribution of the Primary Factors Associated With Early Mortality in Each Cohort
| AMULTEI cohort (n=72) | Historical cohort (n=155) | OR (95%CI) | P | |
| Prosthetic valve endocarditis | 36.1 | 29.7 | 1.33 (0.74-2.42) | .33 |
| Neurological complications | 19.4 | 29.0 | 0.59 (0.30-1.16) | .25 |
| Heart failure | 48.6 | 51.0 | 0.91 (0.52-1.59) | .74 |
| Severe heart failure | 12.5 | 18.7 | 0.62 (0.28-1.39) | .24 |
| Acute renal failure | 40.3 | 35.5 | 1.23 (0.69-2.18) | .48 |
| Perivalvular involvement | 22.2 | 16.8 | 1.41 (0.71-2.85) | .33 |
| Septic shock | 9.7 | 24.5 | 0.33 (0.14-0.79) | .009 |
95%CI, 95% confidence interval; AMULTEI, Alerta Multidisciplinaria en Endocarditis Infecciosa (Multidisciplinary Alert in Infective Endocarditis); OR, odds ratio.
Data from this study indicate that it is possible to reduce early LSIE mortality. AMULTEI patients were significantly older and had more comorbidity, which is consistent with recent reports in the literature. This most likely caused the high prevalence of enterococcal endocarditis in the AMULTEI cohort, which showed a nearly significant increase in comparison with that in the historical cohort, as these bacteria are often found in elderly individuals, usually those with degenerative heart disease and comorbidities.17 Enterococcal endocarditis therefore carries high mortality.18 In a recently published series of 500 cases of enterococcal endocarditis, annual mortality was 28.9%, significantly higher than for other streptococci.19
In recent years, a larger number of staphylococcal endocarditis cases related to S. viridans have been reported, both in studies in Spain4,5 and in other western countries.20–22 In our series, staphylococci represented close to 30% to 35% of cases in both cohorts. It appears that there has been a nonsignificant trend toward a higher incidence of early prosthetic endocarditis in recent years, which is difficult to explain, but as older patients age, their comorbidities and the greater aggressiveness of hospital organisms may play an essential role.
The AMULTEI strategy has helped to increase awareness of LSIE in different areas of the hospital, even in those not directly related to the condition, particularly the emergency room, which allows emergency staff to perform appropriate measures for early diagnosis and treatment. Thus, in the AMULTEI cohort, fewer negative blood cultures (6.9% vs 14.8%; P=.09) were obtained, which we believe was primarily related to higher clinical suspicion and the reduced use of empirical antibiotic therapy before blood extraction in the emergency room, facilitating effective antibiotic therapy guided by sensitivity testing.
Furthermore, the introduction of a microbiological alert in cases of persistent bacteremia by infective endocarditis-causative organisms, personally informing the physician in charge, has contributed to effective early therapy, an important factor for the control of bacteremia and cardioembolism complications10 in patients diagnosed with endocarditis. In fact, there was a trend toward a reduction in neurological manifestations in the recent cohort (19.4% vs 29.0%; P=0.25) and a significant reduction in cases of septic shock (9.7 vs 24.5%; P=.009).
There has been a trend towards greater use of transesophageal echocardiography (73.6% vs 60.8%; P=.06), which might facilitate the diagnosis of intracardiac complications, which are common in this condition and usually require surgery. This trend, together with the increased incidence of prosthetic endocarditis, probably accounts for the increasing trend in the number of surgeries performed during hospitalization observed in the recent cohort.
The data in Table 5 indicate a trend toward an increased number of surgical interventions during hospitalization that was nearly statistically significant; however, this alone was not independently associated with lower mortality in the multivariate analysis. On the other hand, a significantly lower number of patients underwent surgery after a period that we qualitatively defined as a delay in diagnosis and/or surgical indication, which placed these patients in a worse clinical position to tolerate surgery or the postoperative period. In fact, the contrast between the high mortality in emergency surgery compared with elective surgery and how this relates to final hospital mortality is well known,23 making it advisable to treat LSIE patients in centers with cardiac surgery, where early surgery can be performed, if indicated.
The possibility of reducing mortality with broader and earlier indications for surgery has been analyzed in several studies, with generally favorable results.24–28 Two factors may have contributed to the improvement in surgical mortality figures in AMULTEI patients. On the one hand, most surgery was performed early; on the other hand, there was a nearly significant reduction in mortality in early surgery, which could have been related to improved preoperative medical management. Patients at highest risk of complications and those with poor prognosis are those who would most benefit from surgery. Several studies have analyzed prognostic factors in these patients29,30 and have found that prosthetic valve endocarditis, staphylococcal etiology, heart failure,31 neurological disorders, perivalvular complications and central nervous system embolisms influence prognosis, which is consistent with the findings of the present study. Moreover, there is a rapid risk stratification scale32 that has recently been validated and identifies the most severely ill patients (with staphylococcus infection, heart failure, and perivalvular abscess) in the first 72h after admission.
In view of the above, we conclude that the observed reduction in mortality in this study was related to the multidisciplinary approach taken to the diagnosis and treatment of this serious disease. This most likely contributed to rapid diagnosis and early treatment with a decrease in serious complications such as neurological disease, severe heart failure and septic shock, which are invariably fatal in these patients. In establishing this strategy, the collaborations of cardiologists with infectious disease specialists, microbiologists and surgeons play a key role33,34; this is not the only area in which improving and streamlining collaborations among professionals35 have been associated with mortality reduction.36
LimitationsThis study has limitations inherent to its observational design and the use of a historical cohort for comparison (as is commonly used in studies of infectious endocarditis). The number of patients in the second period analyzed (AMULTEI) was lower than in the first period, which was probably why some analyzed aspects did not reach statistical significance. In fact, the number of fatal events in the AMULTEI series was limited to 12.
In addition, we were unable to analyze the time elapsed between the indication for surgery and the performance of surgery, even though a decrease in this interval could be an important prognostic factor.
CONCLUSIONSDespite a trend toward older age and greater comorbidity measured by the Charlson index, mortality was significantly lower in patients treated with the AMULTEI strategy.
FUNDINGThis study was conducted with human resources from the Carlos III Health Institute through the Rio Hortega Scholarship.
CONFLICTS OF INTERESTNone declared.