In the general population, heart events occur more often during early morning, on Mondays, and during winter. However, the chronobiology of death in heart failure has not been analyzed. The aim of this study was to determine the circadian, day of the week, and seasonal variability of all-cause mortality in chronic heart failure.
MethodsThis was an analysis of all consecutive heart failure patients followed in a heart failure unit from January 2003 to December 2008. The circadian moment of death was analyzed at 6-h intervals and was determined by reviewing medical records and by information provided by the relatives.
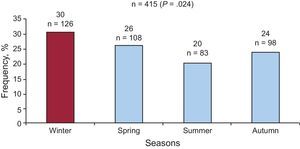
ResultsOf 1196 patients (mean [standard deviation] age, 69 [13] years; 62% male), 418 (34.9%) died during a mean (standard deviation) follow-up of 29 (21) months. Survivors were younger, had higher body mass index, left ventricular ejection fraction, glomerular filtration rate, hemoglobin and sodium levels, and lower Framingham risk scores, amino-terminal pro-B type natriuretic peptide, troponin T, and urate values. They were more frequently treated with angiotensin receptor blockers, beta-blockers, mineralocorticoids receptor antagonists, digoxin, nitrates, hydralazine, statins, loop diuretics, and thiazides. The analysis of the circadian and weekly variability did not reveal significant differences between the four 6-h intervals or the days of the week. Mortality occurred more frequently during the winter (30.6%) compared with the other seasons (P = .024).
ConclusionsAll cause mortality does not follow a circadian pattern, but a seasonal rhythm in patients with heart failure. This finding is in contrast to the circadian rhythmicity of cardiovascular events reported in the general population.
Keywords
A higher incidence of heart events during the early morning hours has been described, mainly due to circadian variation that affects the functioning of the cardiovascular system. Accentuation of the activity of the sympathetic nervous system after awakening and, consequently, activation of neurohormones and thrombosis, may play an important role. Increased peaks in the incidence of acute myocardial infarction, myocardial ischemia, out-of-hospital cardiac arrest, sudden death, and paroxysmal atrial fibrillation have been demonstrated in the general population during the early hours after awakening.1–8 However, despite the high prevalence of heart failure (HF), the circadian variations of death in this syndrome remain uncertain. Only 3 studies have analyzed the circadian pattern of death in HF, and they afford different results. Two studies evaluated the sudden death, and one the end-stage HF death. Moser et al9 studied the pattern of sudden death in 566 patients with advanced HF and found a peak in the early morning (from 6:00 a.m. to 11:59 a.m.). In contrast, Carson et al,10 in a multicenter trial with 1153 patients, did not find the morning peak but reported an evening peak of sudden deaths in the ischemic subgroup of patients. More recently, Aronow et al11 reported data from a study on the time of death due to congestive HF after myocardial infarction in patients over 60 years old and the same morning peak was found as for other cardiovascular events in the general population. However, the circadian pattern of all-cause death in HF patients has not been previously determined.
An increased incidence of acute myocardial infarction and sudden death on Mondays has also been previously described in the general population.12–18 However, it is not known if the same weekly distribution occurs in HF patients. Despite new therapeutic approaches, HF still has a poor prognosis. Recognizing the cause and timing of events in these patients may permit a tailored approach for improving prognosis, such as a stricter follow-up in certain months of the year or stress-directed therapies. With this aim, we analyzed the timing of death in a large cohort of patients with HF to assess the distribution of all-cause mortality according to circadian pattern, day of the week, month of the year, and season.
METHODSStudy PopulationThe study population consisted of 1233 consecutive patients followed up in a multidisciplinary HF unit of a university tertiary hospital with a cardiac transplantation program, from January 2003 to December 2008. All patients had symptomatic HF and were treated according to the guidelines of the European Society of Cardiology.19 Patients undergoing heart transplantation during follow-up were excluded from the analysis. The etiology of HF was determined from the clinical history and according to the results of cardiac imaging. The study was approved by the institution's investigation ethics committee and conforms with the principles outlined in the Declaration of Helsinki.20
Study VariablesDemographic, clinical, echocardiographic, and therapeutic data were collected in all patients.
Determination of the Mode and Time of DeathDeaths were divided into the following categories: sudden death, death due to progressive HF, other cardiovascular deaths, and noncardiovascular death according to the definitions proposed by Narang et al.21 Sudden death was defined as a witnessed or unwitnessed death in the absence of pre-existing circulatory failure or other modes of death; as patients resuscitated from a cardiac arrest, in the absence of pre-existing circulatory failure or other modes of death, who died within 24 h; or as similar patients who died during an attempted resuscitation. Deaths due to progressive pump failure were defined by the presence of at least one of the following situations at the time of death: cardiogenic shock, pulmonary edema, HF symptoms or signs requiring continuous intravenous therapy or oxygen administration, and confinement to bed due to HF symptoms. Other cardiovascular deaths included stroke, perioperative, mesenteric infarction, peripheral vascular occlusion, and others. Furthermore, cardiovascular death included sudden death, death due to progressive HF, stroke, and other cardiovascular events. Noncardiovascular deaths included those due to infection, cancer, and others.
A follow-up telephone call was made to all patients at one year after inclusion. In the event of a patient's death, the study investigators retrospectively categorized the mode and time of death. When the patient died in a hospital, data were obtained by reviewing the medical records. When the patient died outside the hospital, we obtained this information through relatives. Since it was not always possible for the family to provide us with the exact time of death, we divided the day into 6-h intervals to ensure that the registered time range of death was correct. In cases in which it was impossible to get in touch with the family, we consulted the mortality register of the Catalan Health Service to determine the date of death. In those patients, we were not able to obtain the circumstances or time of death because these data are not available in the official registry.
Statistical AnalysisDescriptive analyses were performed as the first step. Categorical variables were described by frequencies and percentages, continuous variables by means and standard deviations (SD). Baseline characteristics of patients across the two mortality categories were examined by the chi-square test for categorical variables or Fisher's Exact Test, if necessary. The comparison of continuous variables between groups was carried out using Student t test for unpaired data once normality was demonstrated (Kolmogorov-Smirnov test); otherwise, a nonparametric test (Mann-Whitney U test) was used. The chi-square Test or Fisher Exact Test, if necessary, were used to assess whether different distributions of mortality at different times were statistically significant. All the analyses were performed with SPSS for Windows (version 17) and a 2-sided P-value <.05 was considered statistically significant.
RESULTSGeneral CharacteristicsDuring the study period, 1233 patients were included and followed up for a mean (SD) of 29 (21) months. Of these patients, 37 were transplanted during the follow-up period and were excluded from the analysis. Of the remaining 1196 patients (mean [SD] age, 69 [13] years; 62% male), 418 (34.9%) died. There were 69 (16.5%) sudden deaths, 148 (35.4%) deaths due to progressive HF, 30 (7.2%) other cardiovascular deaths, and 104 (24.9%) non-cardiovascular deaths. In 67 (16%) patients, the mode of death was uncertain.
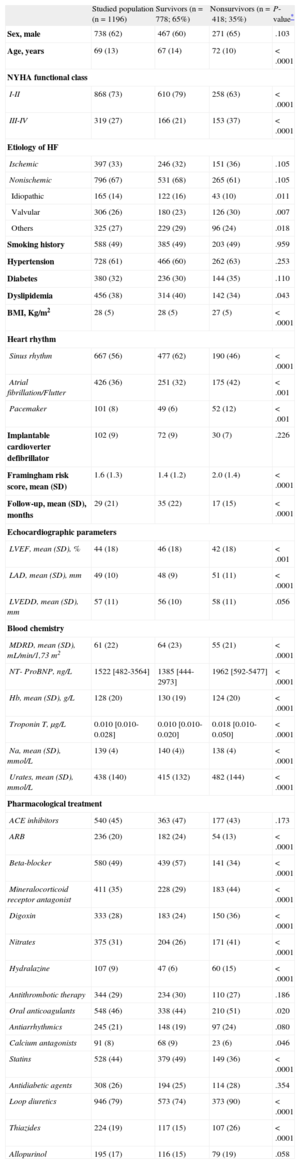
Patients’ clinical characteristics are shown in Table 1, along with differences between survivors and nonsurvivors. Nonsurvivors were more frequently treated with drugs used in more advanced functional classes. It is worth pointing out that there were no differences in survival based on sex, ischemic or non-ischemic etiology of HF, or the following risk factors: smoking history, hypertension, and diabetes mellitus.
Clinical Characteristics of Study Patients Compared by Vital Status
| Studied population (n=1196) | Survivors (n=778; 65%) | Nonsurvivors (n=418; 35%) | P- value* | |
| Sex, male | 738 (62) | 467 (60) | 271 (65) | .103 |
| Age, years | 69 (13) | 67 (14) | 72 (10) | <.0001 |
| NYHA functional class | ||||
| I-II | 868 (73) | 610 (79) | 258 (63) | <.0001 |
| III-IV | 319 (27) | 166 (21) | 153 (37) | <.0001 |
| Etiology of HF | ||||
| Ischemic | 397 (33) | 246 (32) | 151 (36) | .105 |
| Nonischemic | 796 (67) | 531 (68) | 265 (61) | .105 |
| Idiopathic | 165 (14) | 122 (16) | 43 (10) | .011 |
| Valvular | 306 (26) | 180 (23) | 126 (30) | .007 |
| Others | 325 (27) | 229 (29) | 96 (24) | .018 |
| Smoking history | 588 (49) | 385 (49) | 203 (49) | .959 |
| Hypertension | 728 (61) | 466 (60) | 262 (63) | .253 |
| Diabetes | 380 (32) | 236 (30) | 144 (35) | .110 |
| Dyslipidemia | 456 (38) | 314 (40) | 142 (34) | .043 |
| BMI, Kg/m2 | 28 (5) | 28 (5) | 27 (5) | <.0001 |
| Heart rhythm | ||||
| Sinus rhythm | 667 (56) | 477 (62) | 190 (46) | <.0001 |
| Atrial fibrillation/Flutter | 426 (36) | 251 (32) | 175 (42) | <.001 |
| Pacemaker | 101 (8) | 49 (6) | 52 (12) | <.001 |
| Implantable cardioverter defibrillator | 102 (9) | 72 (9) | 30 (7) | .226 |
| Framingham risk score, mean (SD) | 1.6 (1.3) | 1.4 (1.2) | 2.0 (1.4) | <.0001 |
| Follow-up, mean (SD), months | 29 (21) | 35 (22) | 17 (15) | <.0001 |
| Echocardiographic parameters | ||||
| LVEF, mean (SD), % | 44 (18) | 46 (18) | 42 (18) | <.001 |
| LAD, mean (SD), mm | 49 (10) | 48 (9) | 51 (11) | <.0001 |
| LVEDD, mean (SD), mm | 57 (11) | 56 (10) | 58 (11) | .056 |
| Blood chemistry | ||||
| MDRD, mean (SD), mL/min/1,73 m2 | 61 (22) | 64 (23) | 55 (21) | <.0001 |
| NT- ProBNP, ng/L | 1522 [482-3564] | 1385 [444-2973] | 1962 [592-5477] | <.0001 |
| Hb, mean (SD), g/L | 128 (20) | 130 (19) | 124 (20) | <.0001 |
| Troponin T, μg/L | 0.010 [0.010-0.028] | 0.010 [0.010-0.020] | 0.018 [0.010-0.050] | <.0001 |
| Na, mean (SD), mmol/L | 139 (4) | 140 (4)) | 138 (4) | <.0001 |
| Urates, mean (SD), mmol/L | 438 (140) | 415 (132) | 482 (144) | <.0001 |
| Pharmacological treatment | ||||
| ACE inhibitors | 540 (45) | 363 (47) | 177 (43) | .173 |
| ARB | 236 (20) | 182 (24) | 54 (13) | <.0001 |
| Beta-blocker | 580 (49) | 439 (57) | 141 (34) | <.0001 |
| Mineralocorticoid receptor antagonist | 411 (35) | 228 (29) | 183 (44) | <.0001 |
| Digoxin | 333 (28) | 183 (24) | 150 (36) | <.0001 |
| Nitrates | 375 (31) | 204 (26) | 171 (41) | <.0001 |
| Hydralazine | 107 (9) | 47 (6) | 60 (15) | <.0001 |
| Antithrombotic therapy | 344 (29) | 234 (30) | 110 (27) | .186 |
| Oral anticoagulants | 548 (46) | 338 (44) | 210 (51) | .020 |
| Antiarrhythmics | 245 (21) | 148 (19) | 97 (24) | .080 |
| Calcium antagonists | 91 (8) | 68 (9) | 23 (6) | .046 |
| Statins | 528 (44) | 379 (49) | 149 (36) | <.0001 |
| Antidiabetic agents | 308 (26) | 194 (25) | 114 (28) | .354 |
| Loop diuretics | 946 (79) | 573 (74) | 373 (90) | <.0001 |
| Thiazides | 224 (19) | 117 (15) | 107 (26) | <.0001 |
| Allopurinol | 195 (17) | 116 (15) | 79 (19) | .058 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; Hb, hemoglobin; HF, heart failure; LAD, left atrial diameter; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; MDRD, estimated glomerular fraction rate using Modification of Diet in Renal Disease formula; NT–proBNP, amino-terminal pro-B type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
Data are expressed as No. (%), mean (standard deviation) or median [interquartile range].
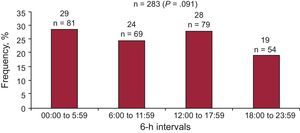
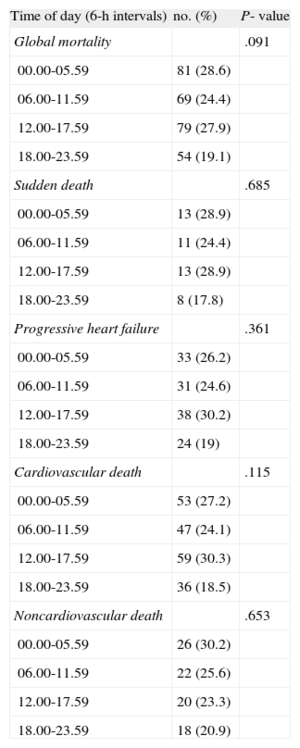
Time of death could be determined within a 6-h interval in 283 of the 418 nonsurvivors (68%). Analysis of the timing of death did not reveal significant differences in distribution between the four 6-h intervals (Figure 1). No significant differences in clinical characteristics were observed for the patients with a known time of death compared with patients with an unknown time of death. The distribution might vary by different factors; therefore, we examined events within the prespecified categories of sudden death, death due to progressive HF, cardiovascular death, noncardiovascular death, ischemic or non-ischemic groups, body mass index, New York Heart Association functional class, and left ventricular ejection fraction. No significant differences in the hourly distribution of deaths were observed in any of these groups (P ≥ .05 for all comparisons). Most relevant data are shown in Table 2.
Subgroup Analysis of the Circadian Pattern of Mortality
| Time of day (6-h intervals) | no. (%) | P- value |
| Global mortality | .091 | |
| 00.00-05.59 | 81 (28.6) | |
| 06.00-11.59 | 69 (24.4) | |
| 12.00-17.59 | 79 (27.9) | |
| 18.00-23.59 | 54 (19.1) | |
| Sudden death | .685 | |
| 00.00-05.59 | 13 (28.9) | |
| 06.00-11.59 | 11 (24.4) | |
| 12.00-17.59 | 13 (28.9) | |
| 18.00-23.59 | 8 (17.8) | |
| Progressive heart failure | .361 | |
| 00.00-05.59 | 33 (26.2) | |
| 06.00-11.59 | 31 (24.6) | |
| 12.00-17.59 | 38 (30.2) | |
| 18.00-23.59 | 24 (19) | |
| Cardiovascular death | .115 | |
| 00.00-05.59 | 53 (27.2) | |
| 06.00-11.59 | 47 (24.1) | |
| 12.00-17.59 | 59 (30.3) | |
| 18.00-23.59 | 36 (18.5) | |
| Noncardiovascular death | .653 | |
| 00.00-05.59 | 26 (30.2) | |
| 06.00-11.59 | 22 (25.6) | |
| 12.00-17.59 | 20 (23.3) | |
| 18.00-23.59 | 18 (20.9) |
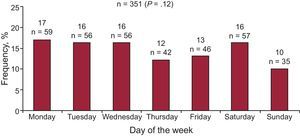
The day of the week was known for 351 of the 418 (84%) deaths. There were no differences in total mortality according to day of the week (Figure 2) or when analyzing the different subgroups of sudden death, death due to progressive HF, cardiovascular death, noncardiovascular death, ischemic or nonischemic etiology of HF, body mass index, New York Heart Association functional class, or left ventricular ejection fraction (P ≥ .05 for all comparisons). There were also no differences when the sub-analysis was performed based on age (younger or older than 65 years; P=. 425).
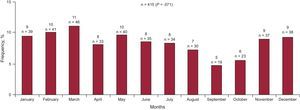
Monthly and Seasonal AnalysisMortality (determined in 99.3% of patients) occurred more frequently during the winter (30.6%), from January to March (Figure 3); only the pairwise comparison (summer-winter) with Bonferroni correction was statistically significant (P=.024) (Figure 4). The same seasonal frequency distribution of deaths was also observed when all patient subgroups were analyzed.
Time-to-death was not an influencing factor: there were no differences in follow-up time between levels of each exposure variable (circadian, day of the week, monthly, and seasonal).
DISCUSSIONCircadian Rhythm of MortalityTo our knowledge, this is the first study evaluating the circadian pattern of death in patients with all New York Heart Association functional classes (I-IV) of HF. We did not observe circadian variability in overall mortality among the four established hourly intervals. In addition, the sub-analysis of the circadian distribution of cardiovascular mortality of this population showed a similar distribution, without peaks of death in any time interval.
These results contrast with the hourly peaks of cardiovascular events found in the general population. Large population studies have shown a non-uniform circadian distribution of heart events with a morning peak (between 6:00 a.m. and 11:59 a.m.).1–8 Moreover, a smaller secondary peak in the late afternoon (between 18:00 p.m. and 23:59 p.m.) was also described,1,2,6 suggesting a bimodal peak of events. The morning peak of heart events has been attributed to the accentuation of the activity of the sympathetic nervous system after awakening. In contrast, the reasons for a second late evening peak are less obvious and may reflect a different process.
Since the sympathetic nervous system is characteristically activated in HF, causing chronic neurohormonal stimulation,22 we expected to find a more uniform distribution of the circadian pattern of mortality. In this syndrome, the constant neurohormonal activation could be responsible for the disappearance of the morning peak of mortality described in the general population. Furthermore, it is possible that HF therapies, which generally create a neurohormonal blockade, and beta-blockers blunt the increased neurohormonal activity and other catecholamine-related changes that take place in the early morning hours.
Weekly Rhythm of MortalityThe prevalence of cardiovascular events on Mondays, compared to the rest of the week, is attributed to the shift from a period of nonscheduled to scheduled activity.12 The onset of acute myocardial infarction peaks on Monday, primarily in the working population,13 supports the hypothesis that external triggering factors may play a role in the acute causation of this disease, probably also stimulating sympathetic nervous system activity. Furthermore, when analyzing the weekly pattern of sudden death, the increase in mortality on Mondays is even more pronounced in patients ≤65 years than in older, apparently retired patients.14 In our HF population, however, we did not see a weekly peak of events in the two age groups (under and over 65 years), which may support the hypothesis that permanent sympathetic activation and the specific treatment of these patients causes the disappearance of event peaks, normalizing their distribution. The employment situation of our patients was not recorded, but most patients were of retirement age and we must take into account that patients with severe HF are not usually active. However, we cannot draw definitive conclusions.
Seasonal Rhythm of MortalityWe found a significant seasonal variation with a maximum incidence of mortality in winter. This distribution is similar to the seasonal pattern of mortality previously described for cardiovascular events in the general population.14,23–25 Moreover, when a HF population was studied, the same seasonal pattern of mortality has been reported.15–18 Several physiological mechanisms may be involved in this seasonal variation.17 First, hemodynamic stress and neurohumoral activation related to a decrease in the ambient temperature may exacerbate HF. Second, myocardial ischemia and arrhythmias induced by temperature decline could increase the risk of HF decompensation. Third, there may be a higher incidence of respiratory infections in winter that may exacerbate HF. Fourth, winter peaks of alcohol consumption may aggravate HF by depressing myocardial contractility and inducing atrial fibrillation.26,27 Furthermore, it should be noted that the elderly are less able to regulate body temperature and are more susceptible to respiratory infections, which could make them more prone to decompensation than other age groups. To further assess the seasonal differences, we must recall that HF admissions tend to be lower in big urban hospitals during summer due to holiday stays in rural or shore localities; therefore, an analysis of in-hospital vs out-of-hospital death would be interesting for future studies.
Study LimitationsOur study has some limitations. First, this is a retrospective design; no autopsies were performed to establish the cause of death and there is insufficient power to reject the null hypothesis for all different modes of death. The time of death could be determined at 6-h intervals in 68% of the patients. Nevertheless, no significant differences in clinical characteristics of the patients with a known or unknown time of death were observed. Other nonrecorded variables, such as comorbidities or employment situation, may play a role in the reported results. Finally, we have to comment on the surprisingly low proportion of patients receiving angiotensin-converting enzyme inhibitors, angiotensin receptor blocker and beta-blockers. The HF unit in which the studied population was attended is a specialized unit to which evolved HF patients are referred. Frequent explanations for not using these drugs were hypotension in patients with needs of high doses of diuretics or ischemic patients with needs of high doses of vasodilators, advanced renal failure, or hyperkalemia.
Clinical ImplicationsDespite these limitations, our findings may have important consequences in the clinical management of these patients. Stricter monitoring of these patients should be implemented in winter to detect and correct exacerbations at an early stage. Furthermore, encouraging vaccination may decrease the worsening of HF related to infections in winter.
ConclusionSIn this large series, HF patients showed no increased peaks of circadian and day of the week mortality, in contrast to the pattern of cardiovascular events in the general population. This finding supports that important physiopathological changes occur in HF and may provide additional insight into the mechanism of death and possible means of prevention. Finally, a marked peak of mortality in winter has been described, suggesting that stricter monitoring of these patients during this time of the year may improve their prognosis.
FUNDINGThis work has received financial support from Instituto de Salud Carlos III, REDINSCOR (Red de investigación clínica y básica en insuficiencia cardiaca) RD06/0003/0015 and RETICS (REdes Temáticas de investigación Cooperativa en Salud): Red Cardiovascular (RD12/0042/0047), Ministerio de Economía y Competitividad (Juan de la Cierva, JCI-2012-14025), and TerCel (Red de Terapia Celular) (RD12/0019/0029).
Conflicts of InterestNone declared.