Preoperative measures aiming to achieve a correct balance between pulmonary flow and systemic flow is of paramount importance in the preoperative stabilization of patients with single ventricle (SV) physiology and in congenital heart diseases with duct-dependent systemic or pulmonary circulation. The physiologic drop in pulmonary vascular resistance (PVR) observed after birth leads to pulmonary over-circulation and to systemic hypoperfusion with increased risk of pulmonary edema, necrotizing enterocolitis,1 and brain stroke.2
Nitrogen inhalation therapy reduces the fraction of inhaled oxygen inducing hypoxic vascular vasoconstriction and hence PVR, thus preventing pulmonary over-circulation3 and improving cardiac output and cerebral blood supply.4 Nevertheless, the use of nitrogen therapy remains controversial. Moreover, no study has evaluated the direct effect of nitrogen inhalation in decreasing pulmonary over-circulation.
Lung ultrasound (LUS) has proven to be a useful tool to monitor pulmonary edema in newborns and children with congenital heart disease.5,6
We report our institutional experience in the management of patients with a combination of afterload reduction and inhaled subatmospheric therapy. We included patients with SV physiology and systemic ductal dependent circulation admitted to our institution between 2016 and 2018.
After appropriate stabilization at the delivery room, and upon arrival at the neonatal intensive care unit, patients were started on prostaglandin infusion, milrinone infusion, and subatmospheric therapy. Prostaglandin infusion was initiated at 0.01 μg/kg/min, interquartile range [0.005-0.02] and milrinone at a dose ranging between 0.15 and 1 μg/kg/min. Subatmospheric therapy was instituted by adding nitrogen, targeting a fraction of inhaled oxygen of 15% to 20% through a high-flow nasal cannula or noninvasive ventilation depending on the patient's condition.
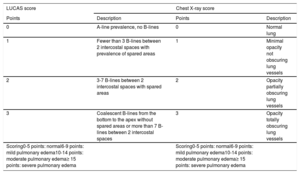
Pulmonary overflow was assessed with the LUCAS score (Lung Ultrasonography in CArdiac Surgery) score, a LUS score created by our group (Table 1). Six sections for each patient were studied. LUS procedures were performed prior to cardiopulmonary bypass and postcardiopulmonary bypass: at 12, 24, 36, 48, and 72 hours later. This score was compared with a chest X-ray score (Table 1). Chest X-ray was done in all patients prior to and immediately after surgery.
LUCAS and chest X-ray scores
| LUCAS score | Chest X-ray score | ||
|---|---|---|---|
| Points | Description | Points | Description |
| 0 | A-line prevalence, no B-lines | 0 | Normal lung |
| 1 | Fewer than 3 B-lines between 2 intercostal spaces with prevalence of spared areas | 1 | Minimal opacity not obscuring lung vessels |
| 2 | 3-7 B-lines between 2 intercostal spaces with spared areas | 2 | Opacity partially obscuring lung vessels |
| 3 | Coalescent B-lines from the bottom to the apex without spared areas or more than 7 B-lines between 2 intercostal spaces | 3 | Opacity totally obscuring lung vessels |
| Scoring0-5 points: normal6-9 points: mild pulmonary edema10-14 points: moderate pulmonary edema≥ 15 points: severe pulmonary edema | Scoring0-5 points: normal6-9 points: mild pulmonary edema10-14 points: moderate pulmonary edema≥ 15 points: severe pulmonary edema | ||
LUCAS, Lung Ultrasonography CArdiac Surgery.
The assessment of systemic cardiac output was based on arterial blood analyses, urine output, serum creatinine, need for inotropic support (excluding milrinone), and the presence of necrotizing enterocolitis or brain stroke (Table 2).
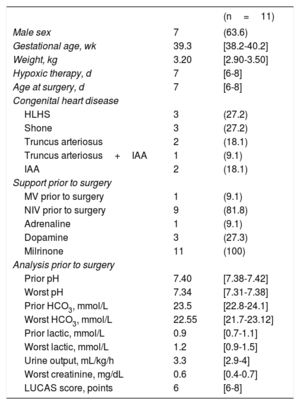
Descriptive patient data before surgery
| (n=11) | ||
|---|---|---|
| Male sex | 7 | (63.6) |
| Gestational age, wk | 39.3 | [38.2-40.2] |
| Weight, kg | 3.20 | [2.90-3.50] |
| Hypoxic therapy, d | 7 | [6-8] |
| Age at surgery, d | 7 | [6-8] |
| Congenital heart disease | ||
| HLHS | 3 | (27.2) |
| Shone | 3 | (27.2) |
| Truncus arteriosus | 2 | (18.1) |
| Truncus arteriosus+IAA | 1 | (9.1) |
| IAA | 2 | (18.1) |
| Support prior to surgery | ||
| MV prior to surgery | 1 | (9.1) |
| NIV prior to surgery | 9 | (81.8) |
| Adrenaline | 1 | (9.1) |
| Dopamine | 3 | (27.3) |
| Milrinone | 11 | (100) |
| Analysis prior to surgery | ||
| Prior pH | 7.40 | [7.38-7.42] |
| Worst pH | 7.34 | [7.31-7.38] |
| Prior HCO3, mmol/L | 23.5 | [22.8-24.1] |
| Worst HCO3, mmol/L | 22.55 | [21.7-23.12] |
| Prior lactic, mmol/L | 0.9 | [0.7-1.1] |
| Worst lactic, mmol/L | 1.2 | [0.9-1.5] |
| Urine output, mL/kg/h | 3.3 | [2.9-4] |
| Worst creatinine, mg/dL | 0.6 | [0.4-0.7] |
| LUCAS score, points | 6 | [6-8] |
HCO3, bicarbonate; IAA, interrupted aortic arch; HLHS, hypoplastic left heart syndrome; LUCAS, Lung Ultrasonography CArdiac Surgery; MV, mechanical ventilation; NIV, noninvasive ventilation.
Qualitative variables are expressed as frequencies (%) and quantitative variables as the median [interquartile range].
Eleven consecutive patients were included in this sample. The main data are shown in Table 2.
There was no correlation between scores on LUS and chest X-ray before surgery (P=.57) and after surgery (P=.62).
Blood analyses were evaluated according to the worst result prior to surgery (Table 2). Before surgery, all patients maintained adequate systemic cardiac output, urine output, and creatinine levels. There were no episodes of necrotizing enterocolitis.
Assessment of pulmonary over-circulation through LUS showed low values and only 1 patient required mechanical ventilation before surgery.
Regarding outcomes, length of mechanical ventilation after surgery was 3 days [IQR, 3-5]. One patient had a brain stroke (9.1%). Overall mortality after surgery was nil.
There was a strong correlation between inotrope score and LUCAS score (P≤.05 at all evaluated times). Higher LUCAS scores were correlated with more inotropic medication.
Blood tests showed no differences in partial pressure of oxygen (PaO2) or partial pressure of carbon dioxide (PaCO2) in patients with lower or higher LUCAS scores (P> .05)
The ratio of pulmonary/systemic blood flow in SV physiology patients directly depends on the ratio between existing vascular resistances. Immediately after birth, newborns with SV physiology usually remain well balanced since circulation remains as in fetal life, with high PVR and low systemic vascular resistance. This balance is altered by the physiologic drop in PVR that occurs after the first days of life. To avoid pulmonary over-circulation and systemic hypoperfusion, several strategies have been evaluated including permissive mild acidosis, hypoventilation, therapeutic hypercarbia, and subatmospheric gas therapy.
As blood flow follows the path of least resistance, it is extremely important to decrease systemic vascular resistance in order to avoid systemic hypoperfusion. The rapid use of both milrinone and subatmospheric therapy could play an important role in balancing it.
Assessment of cardiac output and pulmonary over-circulation in SV patients is challenging, and invasive monitoring devices are difficult to use. In our study, we have shown that LUS is a good noninvasive tool to monitor pulmonary overflow. As pulmonary edema evolves, the normally appearing LUS includes multiple A-lines that change to multiple B-lines, which will then evolve to coalescent B-lines.6 In previous studies in children,5,6 LUS has proven to be useful to monitor lung edema.
The use of invasive monitoring systems and frequent laboratory sampling including mixed venous saturation, lactate, and acid-base status are the commonly used invasive methods to assess cardiac output and appropriateness oxygen utilization. Based on our clinical and laboratory assessment, we have shown in our series that those patients with a lower LUCAS score achieved better systemic oxygen delivery. In our sample, in those patients with higher LUCAS scores, diuretic treatment was increased to help decrease pulmonary overload. The need for preoperative mechanical ventilation is associated with overall worse outcomes. In our sample, only 1 patient (1/11) required mechanical ventilation because of hemodynamic instability due to supraventricular tachycardia.
In our patient population, we found no relationship between PaO2 nor PaCO2 values and LUCAS score. A plausible explanation is that the SV physiology of the underlying cardiac disease might have acted as a confounding factor.
In conclusion, in the present study, we have proven that the combination of subatmospheric therapy using nitrogen and the use of systemic vasodilatory therapy with milrinone is a safe and effective strategy to balance circulation in patients with SV physiology and/or systemic duct-dependent circulation.
.