Keywords
INTRODUCTION
Thoracic aortic disease has traditionally been treated by surgery. Initially, mortality ranged between 5% and 20%.1 Later, various groups began to implant aortic endografts (AE), with a mortality of 0% to 16%.2-4 One of the problems with these procedures is the large size of the implant systems, which require surgery for vascular access and closure. Surgical complications with exposure of the femoral artery in AE implantation procedures are not uncommon and include hematoma, seroma, and infection.5
Percutaneous techniques have been developed to close the access site in AE implantation procedures. The purpose of this study was to describe our experience and immediate- and long-term results with endovascular percutaneous closure devices (Prostar XL®) in the treatment of thoracic aortic disease with endografts.
METHODS
Study Design
The thoracic aorta unit of our hospital was created in December 2001. Among the patients selected by the thoracic aorta unit for implant of an AE (n=26), we prospectively included in the study 10 consecutive patients who underwent fully percutaneous implantation with posterior suture of the access site using two Prostar XL® devices (Perclose, Abbott Vascular Devices, Redwood City, California).
Inclusion and Exclusion Criteria
The protocol for percutaneous treatment has been used at our hospital since June 2004. All patients with thoracic aortic disease eligible for endovascular treatment with AE implant undergo computed tomography (CT) with three-dimensional reconstruction and arteriography to assess anatomy. In addition to data on the aortic disease, these techniques provide information on the diameter of the common femoral artery and its bifurcation, as well as the presence of calcium and/or atheromatous plaques. The exclusion criteria were severe vascular disease, presence of iliac or femoral arteries with a diameter <8 mm or partially occluded, or patients with prior iliofemoral revascularization surgery.
Experimental Methods
Description of the Device
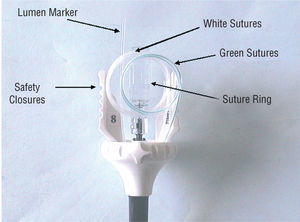
The Prostar XL® device is designed to apply 2 polyester sutures to close femoral artery access sites after diagnostic or therapeutic catheterization procedures (Figure 1). The device has flexible sheaths that house 4 suture needles, a guidewire to control needle deployment around the access site, and a barrel that receives the needles. The sheath measures 38 cm and the distal tip is J-shaped. The barrel contains a lumen marker and the needle guidewire offers the intraluminal access point. The lumen marker provides an outlet for blood from the femoral artery that ensures correct placement of the device. The barrel turns independently of the central core and is designed to prepare the subcutaneous route. The barrel is rotated by pressing the safety closures that protrude from the handle.
Figure 1. Prostar XL® device: device components (see text for details).
Placement Technique
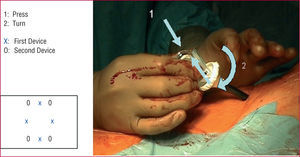
All procedures were performed in the interventional cardiology laboratory under general anesthesia. Following access to the femoral artery using the modified Seldinger technique (puncture of the anterior arterial wall), 2 devices are placed at the puncture site. The first is inserted using the conventional technique guided by a standard 0.038-inch guidewire and is left in the femoral artery after implantation of the 2 sutures. The artery is recanalized with a guidewire and the device is withdrawn. The second device is then inserted, implanting the 2 sutures after turning it with respect to the site where the first Prostar XL® had been placed (Figure 2). The access site is gradually dilated to 20-22 French. The aortic endoprostheses are subsequently implanted and upon completion of the procedure, hemostasis is performed by closing the sutures of the 2 devices with a knot pusher. All patients are anticoagulated during the procedure with 10 000 U of intravenous sodium heparin.
Figure 2. Placement of the second Prostar XL® device (see text for details).
Study Objective and Follow-Up
In the assessment of the immediate outcome, we considered a successful procedure to be complete percutaneous completion with adequate hemostasis and no complications. Follow-up consisted of physical examinations at 24 and 48 h post-procedure and at 6 months by a vascular surgeon. If these examinations detected a new femoral murmur, color Doppler of the femoral artery was performed.
RESULTS
During the study period, 11 patients were treated by endoprosthesis placement, 10 of them percutaneously. The other patient was excluded because of prior iliofemoral revascularization surgery and a surgical approach was used.
The median age was 66 years (35-79 years); 7 (70%) were men. A median of 3 endoprostheses were implanted. The indications included 6 arteriosclerotic aneurysms and 4 type B dissections.
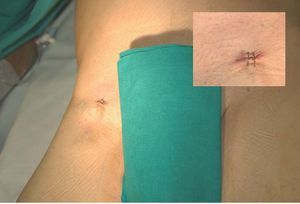
After implanting the endoprostheses, hemostasis was successfully achieved in 9 cases (90%). In the case considered to have failed, the 2 sutures from each vascular closure were initially implanted without complications, but acute occlusion of the femoral artery occurred, secondary to intimal tearing caused by handling the endoprosthesis. Reconversion to surgery was performed without subsequent complications. In the successfully completed cases, no blood transfusion was required. In 1 case, manual compression was needed for 15 min after percutaneous closure to achieve adequate hemostasis. Figure 3 shows the final outcome in 1 of the patients included in the study.
Figure 3. Final result in one of the patients included in the study. The upper right box provides a close-up of view of the access site after closure.
In all the cases considered to be successful, physical examination of the femoral arteries was normal at hospital discharge. After a mean follow-up of 8 months, 2 patients underwent Doppler study of the femoral arteries to investigate murmurs. The examination was normal in 1, and in the other, a femoral pseudoaneurysm was detected, which resolved with compression.
DISCUSSION
The results of our study show that percutaneous access for AE implantation is feasible if the patient is adequately characterized before the procedure. In our experience, 2 aspects are key: a) detailed anatomic study of the femoral and iliac vascular territory, and b) adequate placement of the 2 arterial closure devices.
Studies evaluating complete percutaneous access in endograft implants are limited. Most of them use a single vascular closure device and have been conducted mainly in patients with abdominal aorta disease. Our results in the thoracic aorta are comparable to reported previously reported valves, with success rates ranging from 70% to 95%, according to the type of patient selected.6,7 Haas et al carried out 13 procedures in 12 patients, with 100% success using devices that required arterial dilations of up to 16 Fr.7 In 1 case, they performed dilation of the access site to 22 Fr, followed by successful closure using the same technique as ours with 2 Prostar devices (10 and 8 Fr). Torsello et al conducted a randomized study comparing surgery-based vascular access (n=15) to percutaneous access (n=15) with vascular closure using a single 10 Fr Prostar device.5 The success rate in this study was very high (>90%) and similar in both groups, although the percutaneous approach shortened the surgery and time to ambulation. Recently, Morasch et al evaluated the complications of using a single 10 Fr Prostar device to close 12-18 Fr orifices in abdominal aorta endoprosthesis implantation, with poorer results than those obtained in our study.8 Complications occurred in 19%: 2 patients presented occlusion of the femoral artery and 7, considerable arterial bleeding. In 7 of the 9 complications, reconversion to surgery was necessary, and the authors concluded that percutaneous access can be successfully used in most patients, although always at a hospital equipped with vascular surgery.
These studies show that several factors are related to failure of the device, such as team experience, femoral artery calcification, puncture of a groin with previous scars, obesity, and small-diameter femoral arteries.6
Some authors indicate that the use of 2 devices instead of one may contribute to failure of closure because of crossing of the knots and tearing of the vascular wall when the knots descend.5 In all our patients, vascular closure was performed with 2 devices, and in only 1 patient (who required manual compression for 15 min) only 3 of the 4 knots could be introduced, due to tangling of two of them.
In conclusion, our initial experience with complete percutaneous access for AE implantation in thoracic aortic disease by a double Prostar XL® vascular closure device shows that the procedure can be successfully completed and adequate hemostasis can be obtained in a high percentage of cases. Although larger series are needed to confirm these findings, our long-term results are promising. Proper selection of patients according to femoral anatomy, and correct positioning of the devices are key to achieving these results.
Correspondence: Dr. C. Morís de la Tassa.
Hospital Universitario Central de Asturias.
Celestino Villamil, s/n. 33006 Oviedo. España.
E-mail: cesar.moris@sespa.princast.es
Received September 14, 2005.
Accepted for publication January 19, 2006.