Keywords
Giant cell myocarditis (GCM) is a rare and serious illness, probably of autoimmune origin, histologically characterized by the presence of giant multinucleated cells along with extensive inflammatory infiltrates with large areas of necrosis.1 Until 1997, barely 80 isolated cases had been published and 2 limited series.2 In Spain, only 3 cases have been published as of the date.3-5
We describe a case of GCM whose course required that the patient undergo cardiac transplant as the only therapeutic alternative, the definitive diagnosis being reached via the anatomical study of the explanted heart.
CLINICAL CASE
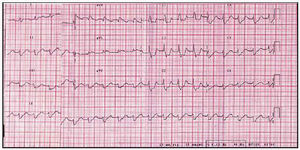
50-year-old patient, without significant clinical history. Hours before admission, the patient went to his physician complaining of a sensation of dyspnea, without fever or symptoms attributable to an infectious process; he was prescribed antibiotic treatment and a bronchodilator. The night of his admission to the hospital he had decubitus intolerance. He went to the emergency room the following morning due to a pre-syncopal episode. Clinical and radiological findings revealed acute pulmonary edema. An ECG was performed (Figure 1) that showed right branch block, anterior hemi block of the right branch, and 1st degree AV block. The occurrence of a complete intermittent AV block that did not require electrical stimulation was noted. The patient was transferred to the intensive care unit and treated with diuretics, IV inotropic drugs (dobutamine), and vasodilators. Electrocardiogram showed a non-dilated left ventricle (diastolic diameter 46 mm), with a severely depressed ejection fraction (25%). Catheterization with coronary angiography was performed and did not reveal significant heart disease, and ventriculography showed a 22% ejection fraction with severe anterolateral and diaphragmatic hypokinesia and slight anterobasal and apical hypokinesia (Figure 2). A series of enzyme tests was normal except for a slight increase in troponin T.
Fig. 1. ECG showing sinusoidal tachycardia with 1st degree AV block, complete block of the right branch, and anterior hemi block of the left branch.
Fig. 2. Ventriculography in right anterior oblique projection in systole and diastole. The ejection fraction is severely depressed, with contraction of the apex and the baseline anterolateral region. The edges have been outlined for improved clarity.
The patient improved enough to be transferred to the floor again, 10 days after admission, but his condition worsened during subsequent days and he was readmitted to the CICU with acute pulmonary edema and cardiogenic shock. A right catheterization was performed and revealed elevated filling pressures (pulmonary capillary pressure 27 mm Hg, mean right atrial pressure 16 mm Hg), and severely reduced cardiac output (3.1 L/m, cardiac index 1.68 L/m/m²), despite treatment with dobutamine and diuretics. Far from improving, the patient´s condition progressively worsened, and the patient was transferred to our hospital to undergo cardiac transplantation.
The patient was admitted to our center 28 days after the initiation of symptoms. An intra-aortic balloon was placed, improving his clinical parameters, and achieving a negative hydric balance and hemodynamic stabilization. Physical examination on admission revealed that the patient´s heart was well perfused but cloudy, with an arterial pressure of 100/75 mm Hg with dobutamine perfusion. The arrhythmic arterial pulse with extrasystoles was 96 beats/minute. An increase in central venous pressure was seen, as well as a gallop in the third and fourth left heart sounds. No murmurs were detected. Hepatomegaly 6 cm from the costal border was seen, and there was no edema.
An ECG was obtained that revealed a sinus rhythm with 1st degree atrioventricular block, complete block of the right branch, and low voltage. Chest x-ray showed acute pulmonary edema. On echocardiogram, the left ventricle was dilated with and ejection fraction <20%. Hemogram and biochemical studies did not show any significant changes.
The patient was placed on the urgent transplant list on the third day after admission, and a transplant was performed the same day. His post-operative course was without complications. He received induction immunodepressor therapy with anti-myocite gamma globulin. The maintenance therapy consisted of triple therapy with cyclosporine, micophenolate mofetil, and prednisone. The patient had a moderate (grade 3A) rejection episode as revealed by biopsy in the second week post-transplant; this was treated with steroid shock, with complete resolution by biopsy in the fourth week without the reappearance of giant cells on any of the followup biopsies. As of this report (6 months post-transplant), the patient is leading a normal life, without dyspnea or other symptoms, with normal graft function on followup echocardiography studies.
Blood work was negative. Polymerase chain reaction technique did not reveal viral genomes in the myocardial samples analyzed.
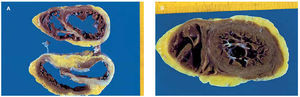
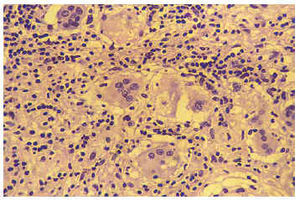
Anatomopathologic macroscopic study of the explanted heart (Figure 3) revealed slight dilatation of both ventricles with replacement of the normal muscle tissue of the basal third of the septum, anterior and lateral walls of the left ventricle, and to a lesser degree of the posterior wall of the left ventricle; the ventricles were scarred in appearance and grayish-brown in color, while the apical third of the heart appeared to be preserved practically in its entirety. The macroscopic changes had a slight effect on the anterior face of the right ventricle. Histological examination (Figure 4) revealed an intense inflammatory infiltrate of lymphocytes and plasma cells, with some eosinofils and abundant multinucleated giant cells. There were areas of serpiginous necrosis and considerable substitution of muscle tissue with fibrous tissue in the previously noted areas. Special tinctures were used to identify acid-alcohol resistant bacilli, which were negative. Serial slices of the coronary arteries showed lesions in the mid descending anterior artery, without signs of instability and not thought to be the cause of the anatomical anomalies noted.
Fig. 3. Anatomical piece of the explanted heart in the basal mid (A) and apical (B) areas. It can be seen that muscle tissue has been replaced by scar tissue in large areas of both ventricles, particularly in the septum and anterior and lateral walls of the left ventricle; nevertheless, the muscle is preserved in most apical portions, corresponding to findings on ventriculography.
Fig. 4. Histological study (hematoxiline-eosina tincture) in which the inflammatory infiltrate composed primarily of lymphocytes and histocytes that can be seen in the multinucleated giant cells can be observed.
DISCUSSION
GCM is a rare disease. Cooper2 reported on the most extensive series: 63 patients from 36 hospitals, citing in the bibliography approximately 90 cases published up to 1997 in English.
In Spain, Ruiz et al3 in 1993 published the case of a woman with GCM accompanied by thymoma, myasthenia gravis, chronic lymphocytic thyroiditis, giant cell myositis, and granulomatous infiltration of the lymphatic nodes of the pulmonary filaments. In 1994, Ariza et al4 reported a case of fatal GCM in a 46-year-old woman with a history of ulcerative colitis. Fernández et al5 in 1999 described a new case of a 57-year-old man who initially had an apical myocardial infarction, with the development of refractory cardiac insufficiency and who finally required a heart transplant. Our case was a 50-year-old patient who presented with cardiac insufficiency and atrioventricular block, who required a cardiac transplant due to the progression from intra-aortic balloon to cardiogenic shock.
GCM is associated with other autoimmune processes in approximately 20% of cases.2,7 In a multicenter compilation study, cardiac insufficiency was the most frequent form of presentration,2 and constituted 75% of cases. Ventricular tachycardia represented 14% of cases, with symptoms including palpitations, syncope, or sudden death. Acute myocardial infarction was present in 6% of cases, and complete atrioventricular block in 5%. The appearance of more than 1 principal manifestation in the course of the disease is common. In the Stanford series,7 the 5 cases developed progressive cardiac insufficiency and ventricular tachycardia. In the Boston series,6 ventricular tachycardia was present in 90% of cases. Clinical diagnosis should be highly suspected in the face of a patient with cardiac symptoms that have recently appeared and are progressively rapidly with signs of intense ventricular dysfunction in the absence of other possible etiologies; endomyocardial biopsy is the definitive diagnostic procedure. It is not unusual for the diagnosis to be the product of the study of the explanted heart.
GCM has a poor prognosis. In the multicenter study, 89% of the patients died or required cardiac transplant within an average of 5.5 months. In the study by Davidoff et al6 a 17% decline in the ejection fraction was noted during an average followup period of 2 years, contrasting with an 8% improvement in the group with lymphocytic myocarditis.
At the present time, treatment with immunosuppressant drugs is favored by various investigators. In the multicenter study, the mean survival rate of untreated patients was 3 months.2 The greatest survival rate, 12.6 months, was obtained in the group of patients treated with cyclosporine in combination with another immunosuppressant agent. The authors indicated the difficulty in evaluating these results, as given the normally fatal course of these patients, only those who have survived for some time without treatment would have the opportunity to receive treatment, creating a bias in the selection of survivors. After the study mentioned, anecdotal cases were published, both as a response and a failure of combined immunodepressor treatment.8,9
Cardiac transplant is, when possible, the principal alternative therapy. This was the case with our patient, and with 34 of the 63 patients reported in the multicenter study, with a survival rate of 74% after an average followup period of 3.7 years.2 GCM can reoccur in the grafts of transplanted patients;10 this occurred in 9 of the 34 transplant patients in the multicenter study, between 3 weeks and 9 years following transplant.
Correspondence: Dr. J. Fernández-Yáñez.
Nicasio Gallego, 19, 6D. 28010. Madrid. Spain.
E-mail: jyanez@jet.es.