The aim of this study was to compare magnetic resonance and gated-SPECT myocardial perfusion imaging in patients with chronic myocardial infarction.
MethodsMagnetic resonance imaging and gated-SPECT were performed in 104 patients (mean age, 61 [12] years; 87.5% male) with a previous infarction. Left ventricular volumes and ejection fraction and classic late gadolinium enhancement viability criteria (<75% transmurality) were correlated with those of gated-SPECT (uptake >50%) in the 17 segments of the left ventricle. Motion, thickening, and ischemia on SPECT were analyzed in segments showing nonviable tissue or equivocal enhancement features (50%-75% transmurality).
ResultsA good correlation was observed between the 2 techniques for volumes, ejection fraction (P<.05), and estimated necrotic mass (P<.01). In total, 82 of 264 segments (31%) with >75% enhancement had >50% single SPECT uptake. Of the 106 equivocal segments on magnetic resonance imaging, 68 (64%) had >50% uptake, 41 (38.7%) had normal motion, 46 (43.4%) had normal thickening, and 17 (16%) had ischemic criteria on SPECT.
ConclusionsA third of nonviable segments on magnetic resonance imaging showed >50% uptake on SPECT. Gated-SPECT can be useful in the analysis of motion, thickening, and ischemic criteria in segments with questionable viability on magnetic resonance imaging.
Keywords
.
INTRODUCTIONMagnetic resonance imaging (MRI) imaging allows analysis of volumes and left ventricular ejection fraction (LVEF) and, via late gadolinium enhancement (LGE), evaluation of the myocardium at risk and determination of the transmurality and extent of myocardial necrosis.1–3 A transmural extent of necrosis greater than 75% of the myocardial wall2,4 or >4.5mm5 is an indicator of nonviable tissue or irrecoverable contractile function after revascularization. In addition, an uptake of < 50% of the maximum uptake of the left ventricle (LV) is being adopted as an indicator of nonviability in single-photon emission computed tomography (SPECT) of myocardial perfusion6 and in fluorodeoxyglucose positron emission tomography (PET) evaluation of metabolism.5 However, there are intermediate values (between 50% and 75% of transmurality for MRI and between 30% and 50% uptake for isotope studies) that raise doubts on the optimal threshold for the more accurate prediction of the possibility of contractile recovery. Thus, some authors have suggested that uptakes of 40% for SPECT with technetium compounds7 and 37% for PET8 are better cutoff values. In addition, on induction of physical or pharmacological stress, these tracer techniques can be used to evaluate the degree of regional systolic wall thickening and the presence of ischemia to further aid the assessment of myocardial viability.9
The aim of this study was to compare the results of stress perfusion MRI and SPECT in a series of patients in the chronic phase of myocardial infarction and to determine the parameters of gated-SPECT that could be useful in the evaluation of viable myocardium.
METHODSPatientsThe present study evaluated 104 nonconsecutive clinically stable patients (mean age, 61 [12] years; 87.5% male) previously diagnosed with myocardial infarction according to international guidelines.10,11 All patients were included in an MRI study of postinfarction ventricular remodeling, approved by the ethics committee of the center (PR-HG-36/2000) and underwent 99mTc-tetrofosmin gated-SPECT at the discretion of the treating physician, with a maximum interval between the 2 scans of less than 1 year.
The clinical and coronary angiographic characteristics of the 104 patients are shown in Table 1. The mean intervals between the acute myocardial infarction and myocardial perfusion SPECT and between SPECT and the MRI scan were 8 (6) months and 6 (4) months, respectively. There were no complications or readmissions due to acute coronary syndrome between the scans.
Clinical, Ergometric, and Coronary Angiograph Characteristics of the Patients
| Age, years | 61 ± 12 |
| Male sex | 91 (87) |
| Diabetes mellitus | 13 (12.5) |
| Hypertension | 53 (50.9) |
| Hypercholesterolemia | 55 (52.9) |
| Smokers | 55 (52.9) |
| Nitrates | 9 (8.7) |
| Beta blockers | 53 (51.0) |
| ACE inhibitors or ARB | 43 (41.3) |
| Previous revascularization | 44 (42.3) |
| Stress test (n=101) | |
| MET | 7.10 ± 2.84 |
| %HRmax | 80.04 ± 11.75 |
| SBPmax | 145 ± 26 |
| HRmax×SBPmax | 18,534 ± 4,888 |
| Angina | 2 (1.9) |
| Coronary angiography (n=86) | |
| Single-vessel disease | 46 (53.5) |
| Double-vessel disease | 23 (27) |
| Triple-vessel disease | 17 (19.5) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; HR, heart rate; MET, metabolic equivalent of task; SBP, systolic blood pressure.
Data are expressed as no. (%) or mean±standard deviation.
MRI were obtained with a 1.5-T system (Avanto, Siemens; Germany) with a 4-element phased-array coil. All sequences were performed with electrocardiographic gating and during a breath hold. Segmented cine-MRI sequences were acquired (TrueFISP; echo time [TE], 1.4ms; repetition time [TR], 55ms; flip angle, 52°; bandwidth, 977Hz/pixel; matrix, 256×212; slice thickness, 8mm) in the short-axis (from the base to the apex of the LV), 2-chamber, and 4-chamber views. Ten minutes after contrast agent administration (gadolinium, 0.15mmol/kg), late enhancement images were acquired with a segmented inversion-recovery sequence (TurboFlash; TE, 1.2ms; TR, 450ms; flip angle, 50°; bandwidth, 1180Hz/pixel; matrix, 192×128; slice thickness, 8mm) after the most appropriate inversion time had been selected to correctly suppress the signal of healthy myocardium and with the same slice positions as those obtained in the cine-MRI images.
The necrotic tissue mass on MRI was calculated by first manually delineating the margins of the hyperenhanced areas in the LGE sequence, then multiplying the specific myocardial mass by the volume estimated from the sum of the hyperenhanced areas by the slice thickness. The transmurality of each myocardial segment was visually evaluated by quantifying the percentage LGE thickness with respect to the total wall thickness, provided that the enhancement occupied more than half of the total wall thickness. Five groups were established according to the degree of transmurality: 1, no LGE; 2, <25% LGE; 3, 25% to <50% LGE; 4, 50% to <75% LGE; and 5, ≥75% LGE.
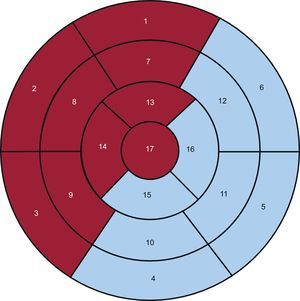
The LV was divided into 17 segments,12 and 2 regions were defined: the anterior (ANT) region, which encompassed the anterior, septal, and apical segments, and the inferolateral (INF-LAT) region, which included the inferior and lateral segments (Fig. 1).
Seventeen-segment polar map that differentiates between the anterior (red background) and inferolateral (blue background) regions. Segments: 1, basal anterior; 2, basal anteroseptal; 3, basal inferoseptal; 4, basal inferior; 5, basal inferolateral; 6, basal anterolateral; 7, mid anterior; 8, mid anteroseptal; 9, mid inferoseptal; 10, mid inferior; 11, mid inferolateral; 12, mid anterolateral; 13, apical anterior; 14, apical septal; 15, apical inferior; 16, apical lateral, and 17, apex.
The variables studied were the end-diastolic and end-systolic volumes and LVEF the necrotic tissue mass in grams and its percentage with respect to the total mass of the LV, necrotic mass estimated by segment, the number of LGE segments weighted by the degree of transmurality, and the degree of segmental LGE transmurality classified as one of the 5 categories described above.
Gated-Single-photon Emission Computed TomographySymptom-limited exercise testing was performed in 101 of the 104 patients. In 16 of these patients who were unable to reach 80% of their peak heart rate or 5 metabolic equivalents of task, dipyridamole was administered at the time of the test.13 For SPECT, a short 1-day protocol was used involving 300MBq of 99mTc-tetrofosmin at stress and 900 MBq of 99mTc-tetrofosmin at rest; the resting test alone with 900 MBq 99mTc-tetrofosmin was performed in 3 patients. Sublingual nitroglycerine was administered to all patients before radionuclide injection for the resting test. Acquisition was begun 30-60min after the stress test and 60-90min after the injection at rest. A dual-head Siemens E.cam gamma camera was used, with adjacent detectors in a 90° (L-mode) configuration, a low-energy and high-resolution collimator, a 99mTc energy window, an acquisition zoom of 1.45, and a 64×64 matrix. The detection arc extended from the 45° right anterior oblique position to the 45° left posterior oblique position, with images taken every 3° at an acquisition time of 27 s per image. For gated-SPECT, an R-R acceptance window of 70% was used at a rate of 8 frames per cardiac cycle. Images were not corrected for attenuation. The tomographic reconstruction of the reorientated heart slices was carried out with OSEM (ordered-subset expectation maximization) iterative reconstruction and with a 0.35/10 Butterworth filter for the stress test (low dose) and a 0.45/10 filter for the resting test (high dose).
The quantitative evaluation of the degree of thickening and the quantitative grading of the perfusion of the wall segments was performed on the polar map of the rest test with QPS automatic software for the perfusion and QGS for gated-SPECT.14,15 In addition to the segment grading, defect extent was calculated by selecting 3 cutoff values for the blackout map: 30%, 40%, and 50%, with the Emory Cardiac Toolbox of Emory University.16
The following variables were studied:
- •
End-diastolic and end-systolic volumes and LVEF.
- •
Percentage extent of the defect in the polar map with an at-rest uptake of <30%, <40%, and <50%.
- •
Percentage uptake in each of the 17 segments, classified into 1 of 5 categories: normal, <30%, 30%-<40%, 40%- <50% and ≥50%.
- •
Regional wall motion, scored on a 6-point scale: 0, normal; 1, mild hypokinesia; 2, moderate hypokinesia; 3, severe hypokinesia; 4, akinesia; and 5, dyskinesia.
- •
Regional wall thickening, scored on a 5-point scale: 0, normal; 1, mildly impaired; 2, moderately impaired; 3, severely impaired; and 4, absent.
A segment was considered to have signs of ischemia on SPECT when the score between the stress and resting tests improved by at least 1 grade. A segment was considered to have normal motion and thickening on gated-SPECT when its score was between 0 and 2.
Statistical AnalysisData on quantitative variables are expressed as means (standard deviations) and intervals, and categorical variables are expressed as percentages or proportions. The Kolmogorov-Smirnov test was used to test the normality of the distribution. The Pearson correlation coefficient was determined for ventricular function variables and volumes obtained with gated-SPECT and MRI and for the intraclass correlation for the amount of MRI transmurality in relation to segmental uptake on SPECT. To compare the quantitative variables by category, the means were compared through an analysis of variance with Bonferroni comparison. A P<.05 was considered statistically significant. A receiver operating characteristic (ROC) curve was plotted for the level of segmental uptake on SPECT and the degree of segmental transmurality on MRI, using as a criterion of viability a transmural LGE of <75% on MRI. Kappa index (k) was calculated between the optimal SPECT threshold of the ROC curve and <75% LGE on MRI.
Three sets of data were analyzed: first, the variables of the 104 patients; second, the variables for each of the 1,768 segments, and, finally, those segments with LGE and those with borderline viability on the MRI. SPSS statistical software package version 15 was used for all analyses.
RESULTSThe correlations between MRI and gated-SPECT for the end-diastolic volume (144 [43] mL vs 149 [56] mL), the end-systolic volume (78 [38] mL vs 88mL), and the LVEF (45% [12%] vs 44% [13%]) were 0.707, 0.762, and 0.707, respectively (P<.05).
The necrotic mass on MRI was 21.7 [13.7] g (16.2% [9.9%] that of the LV). The amount of necrotic mass was <15% that of the total LV mass in 55.8% of the patients, 15%-30% in 35.6%, and >30% in 8.7%. The correlations between the necrotic mass determined with MRI and the extent of necrosis in the SPECT polar map for the thresholds of <30%, <40%, and <50% were 0.498, 0.497, and 0.487, respectively (P<.01). The correlations between the necrotic mass by segment estimated with MRI and the extent of necrosis in the SPECT polar map for the thresholds of <30%, <40%, and <50% were 0.543, 0.523, and 0.512, respectively (P<.01).
Segmental AnalysisWe analyzed 1,768 segments (17 segments from 104 patients).
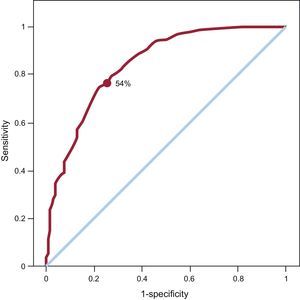
For the SPECT ROC curve of the myocardial perfusion, taking an LGE transmurality of <75% as criteria of viability, the area under the curve was 0.845 (Fig. 2). The optimal SPECT uptake threshold was 54%, which gave the highest sensitivity and specificity (78% and 75.7%, respectively). For this SPECT threshold, the kappa (κ) was 0.409.
Receiver operating characteristic curve plotted for the amount of segmental uptake on single-photon emission computed tomography and magnetic resonance imaging taking as a reference of viability a late gadolinium enhancement transmurality of <75%. Area under the curve=0.845. The uptake point chosen for single-photon emission computed tomography (54%) attempts to maximize both sensitivity (78%) and specificity (74.7%).
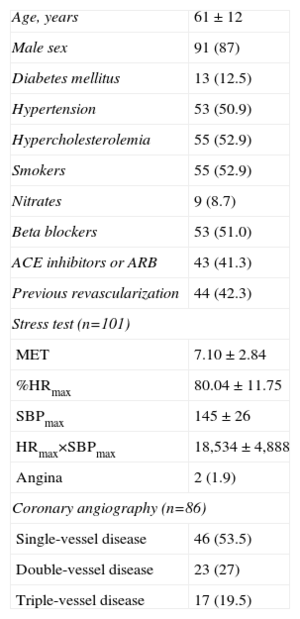
The intraclass correlations between the increase in the amount of LGE transmurality and the reduction in the amount of SPECT uptake in the ANT and INF-LAT regions and in the basal, mid, and apical segments are shown in Table 2. The best correlation between the degree of LGE transmurality and the reduction in the amount of SPECT uptake was found in the apical segments.
Intraclass Correlations Between the Degree of Transmurality of Late Gadolinium Enhancement and the Level of Uptake on Single-photon Emission Computed Tomography
| Segments | R |
| All (n=1768) | −0.420 |
| Anterior region | −0.439 |
| Inferolateral region | −0.380 |
| Basal segments | −0.255 |
| Mid segments | −0.368 |
| Apical segments | −0.450 |
A total of 477 of the 1,768 segments showed LGE, of which 80.1% were located in the ANT region; the remainder (19.9%) were in the INF-LAT region. Of these segments with LGE, 7.5%, 34.8%, and 57.7% had basal, mid-cavity, and apical locations, respectively. The degree of transmurality was <25% in 5% of the segments, 25%-50% in 17.4%, 50%-75% in 22.2%, and >75% in 55.3%. Thus, from the point of view of the MRI, more than half of the segments with LGE did not meet the criteria for viability.
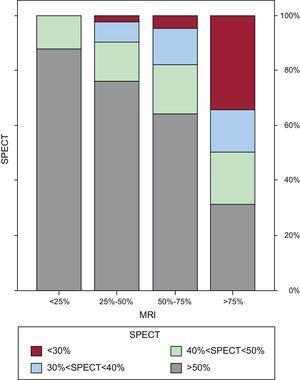
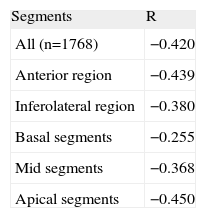
Table 3 and Figure 3 show the relationship between the percentage SPECT uptake and the degree of LGE transmurality expressed in numbers of segments. Of the 264 segments without classic viability criteria on MRI (ie, an LGE transmurality >75%), 82 (31%) had an uptake >50% on SPECT (Fig. 4) and 106 segments (22.2% of the LGE segments) had questionable viability criteria (an LGE of 50%-75%) on MRI. Of these 106 segments, 68 (64%) had an uptake >50% on SPECT, 41 (38.7%) showed normal motion, 46 (43.4%) normal thickening, and 17 (16%) showed criteria of ischemia.
Relationship Between the Percentage Uptake on Single-photon Emission Computed Tomography and the Degree of Late Gadolinium Enhancement Transmurality on Magnetic Resonance Imaging Expressed in Number of Segments
| SPECT | Total | ||||
| <30% | 30% to <40% | 40% to <50% | ≥50% | ||
| MRI | |||||
| <25% | 0 | 0 | 3 | 21 | 24 |
| 25% to 50% | 2 | 6 | 12 | 63 | 83 |
| 50% to <75% | 5 | 14 | 19 | 68 | 106 |
| ≥75% | 90 | 42 | 50 | 82 | 264 |
| Total | 97 | 62 | 84 | 234 | 477 |
MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
Bar graphs showing the relationship between the percentage uptake on single-photon emission computed tomography and the degree of transmurality of the late gadolinium enhancement on magnetic resonance imaging. MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
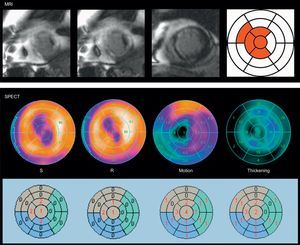
Example of a viability discrepancy between magnetic resonance imaging and single-photon emission computed tomography in a patient with a previous myocardial infarction. The degree of transmurality of the late gadolinium enhancement is >75% in 4 segments (in red), while in the single-photon emission computed tomography polar maps reversibility was seen between stress and rest, in conjunction with normal motion (score, 1-2), and normal thickening (score, 1-2), in those segments, except in the apical segment (score, 4) and apical septum (score, 3). MRI, magnetic resonance imaging; R, rest; S, stress; SPECT, single-photon emission computed tomography.
The results of the present study show that, although the general correlation between MRI and gated-SPECT for the estimation of LV volumes and LVEF is good, there are discrepancies between the techniques in quantification of the extent of necrosis and evaluation of myocardial viability. MRI with LGE has been shown to be superior to SPECT myocardial perfusion imaging for the determination of transmural myocardial necrosis, particularly in subendocardial infractions.17,18 This suboptimal sensitivity of SPECT is explained by its lower spatial resolution. However, the viability threshold of transmural necrosis for a given myocardial segment is not well established. Among other factors, the reason for this lack of a clear cutoff could be that the transmurality of necrosis is not necessarily homogenous throughout the entire myocardial segment. For this and other reasons, the superiority of MRI, even when compared with PET, continues to cause controversy.19,20 Another point that justifies comparison between the 2 techniques is that the use of MRI can present limitations in certain patients, such as those who have pacemakers or automatic implantable defibrillators, lack a regular stable rhythm, are unable to maintain the required breath hold duration, or have renal insufficiency or claustrophobia.
Although we found an acceptable correlation in our series between the overall necrotic mass of the LV estimated by MRI and the extent of necrosis on SPECT, the segment-to-segment comparison between the techniques was less precise. The basal region was the area showing the greatest discrepancy between the degree of LGE transmural necrosis and the percentage uptake on SPECT. This divergence is largely due to problems in the quantification of the amount of isotope uptake in the anteroseptal segments of the basal region in the SPECT polar map. These problems arise because the length of the septum is less than that of the lateral wall of the LV, which means that these segments often show a low percentage uptake that largely corresponds to extracardiac activity.
One of the difficulties of interpreting viability results with both MRI and SPECT is the observation of equivocal or borderline criteria or, in other words, the attainment of “probable results” rather than “definitive results”. Thus, although MRI provides images of a greater spatial resolution, their interpretation can raise doubts about the viability of certain regions. Kim et al.2 observed equivocal or nondiagnostic results in 293 of 804 segments (36%). For these authors, segments with an LGE occupying >75% of the area were considered nonviable and those with an LGE occupying <25% were considered viable. Between these values, the probability of contractile recovery after revascularization is only 50%. Other authors have proposed different cutoff values as viability criteria for MRI, such as a thickness <4.5mm of LGE5 or >3mm of absence of LGE.8 In a series of 26 patients with ischemic cardiomyopathy (ejection fraction, 31% [11%]), Kühl et al.8 found a clear inverse correlation between the degree of LGE and the level of 18F-fluorodeoxyglucose uptake. Through ROC curve analysis, the authors calculated a threshold of 37% PET uptake that best differentiated between viable and nonviable myocardium. By using this value as a reference for PET, the sensitivity and specificity of MRI for the diagnosis of nonviable myocardium were 96% and 84%, respectively. In 2006, Kühl et al.21 used a segmental area of LGE>50% and an uptake of <50% for PET/SPECT as criteria of nonviability in a series of 29 patients with ischemic cardiomyopathy (ejection fraction, 32% [10%]), they obtained a positive predictive value for contractile recovery of 73%, identical for both techniques, while the negative predictive value was higher for MRI (93% vs. 77%).
In our series, we confirmed this negative correlation between the degree of LGE transmurality and the amount of isotope uptake with SPECT, in particular for the apical segments, but we also observed discrepancies between MRI and SPECT. About one-third (31%) of the segments with classic viability criteria on MRI (an LGE >75%) had a percentage uptake of >50% on SPECT. In addition, 22% of the segments with LGE had equivocal viability criteria (an LGE of 50%–75%) on MRI. In these segments, SPECT can aid the viability diagnosis, as 64% had >50% uptake, 39% had normal motion, 43% had normal thickening, and 16% showed reversibility (a stress-rest difference>1) (ischemia). MRI also allows the evaluation of wall thickening, motion and ischemia when performed after stress, which can clarify ambiguous results obtained with LGE evaluation alone.
Segmental study of necrosis and viability does not allow conclusions to be drawn about the general contractile recovery of the LV once the patient is revascularized, because other clinical and coronary angiographic factors play important roles in the prognoses of these patients.22–24 In some series, the minimum amount of viable myocardium required for improvement in general contractile function is considered to be 15%25; however, in a meta-analysis of 29 studies of 4,167 patients, Inaba et al.26 estimated that the amount of viable myocardium necessary for an improved survival after surgical revascularization in ischemic cardiomyopathy was 25.8% with PET, 35.9% with stress echocardiography, and 38.7% with SPECT.
LimitationsPatient inclusion in this study was nonconsecutive and the results obtained only reflect the results and experience of a single center with the scans performed. Moreover, the profile of the patients included was probably less than ideal for the study of myocardial viability because a considerable percentage had an LVEF of >40%. Stress-rest SPECT is more complete than at-rest MRI and the methodology used for segmental evaluation of transmurality is visual with LGE but is performed with an automatic program14,15 that semiquantitatively measures the tracer uptake in the LV polar map with SPECT. In our study, wall motion and thickening were not studied with MRI; however, the correlation of the results obtained with this technique and those obtained through gated-SPECT is good. For example, Whaba et al.27 observed an agreement between the 2 techniques of 80% (κ=0.66) for motion and of 84% (κ=0.70) for thickening in patients with previous infarction. A strict segment-to-segment comparison between the 2 techniques is hampered by the use of a polar map with different characteristics from that used for the MRI. This limitation could be overcome through the use of polar maps that more objectively reflect the extent of LGE. Furthermore, although the distinct segments of each patient are not independent of one another, our statistical analysis focused on segmental comparisons. If we had used attenuation correction in our study, the correlation with the results of MRI would probably have been even closer. Raja et al.28 have shown excellent agreement between SPECT and PET for the evaluation of viability following the use of nitrates and attenuation correction in the SPECT analysis. Finally, the absence of postrevascularization results hindered the estimation of the diagnostic efficacy of MRI and SPECT in the prediction of contractile recovery.
CONCLUSIONSThe correlation between MRI and gated-SPECT for volumes and LVEF and the estimated necrotic mass is good, although the correlation between the degree of transmurality in MRI and SPECT uptake level is suboptimal, particularly in the basal region of the LV. One-third of the segments considered to be nonviable in MRI (an LGE>75%) showed >50% uptake on SPECT. Of the segments considered borderline in the MRI (an LGE of 50%-75%), two-thirds had an uptake of >50% in gated-SPECT, 39% had normal motion, 43.4% had normal thickening, and 16% had ischemia. These results could have clinical implications in those centers that use at-rest MRI as the first-line option for the diagnosis of viability and that obtain inconclusive results, because, in this eventuality, they allow determination of the benefit, if any, that could be provided by a stress-rest gated-SPECT.
CONFLICTS OF INTERESTNone declared.