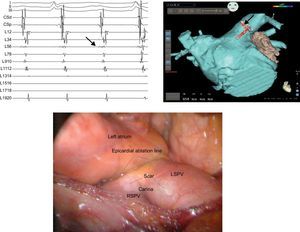
A 70-year-old man presented with a longstanding history of lone atrial fibrillation refractory to medical treatment and pulmonary vein (PV) electrical isolation. Due to left atrial (LA) size (PA, 50mm), he was referred for a hybrid percutaneous endocardial and thoracoscopic epicardial ablation. First, a transseptal puncture and an electroanatomical map was performed, followed by a thoracoscopic video-assisted epicardial ablation. During dissection of the right superior PV roof region (in the area of the right superior ganglionated plexi), the organized atrial fibrillation converted to sinus rhythm. Roof and inferior posterior LA linear ablation was performed using a bipolar linear pen device (Coolrail, Atricure, West Chester, Ohio, United States). Visual inspection of the anterior antrum of the left PV showed the presence of an epicardial scar at the carina and in a distal ostial location in the left superior PV (Fig. 1). Left superior PV isolation was performed in an antral location with the bipolar clam, similar to that described for the right-sided veins (Fig. 1). The roof and inferior linear ablations were continued from the left side under visual control to complete a posterior LA box lesion. After epicardial ablation, PV electrical isolation was confirmed by the absence of PV potentials by means of a LASSO catheter. However, the posterior box was not isolated. The absence of entry block was suggested by the presence of residual local potentials inside the posterior box lesion and the absence of exit block was confirmed by pacing with conduction from the posterior wall to the rest of the LA. Subsequently, detailed endocardial voltage and activation mapping was performed. Endocardial sites with closely or widely split or fragmented potentials on the roof and posterior LA were tagged. Detailed mapping showed the presence of relatively high voltage, and little or no fragmented short-duration local bipolar signals along the endocardial surface of the medial half of the roof line to the right superior PV atrium (Fig. 2). At these sites, endocardial radiofrequency application was performed with local splitting and/or voltage reduction as endpoint. Following these applications, remapping confirmed the entry block in the form of the absence of near field potentials inside the box on the voltage map (Fig. 2). Subsequent aggressive atrial stimulation did not induce atrial fibrillation despite isoproterenol infusion (10μg/min). At 12 months’ follow-up the pacemaker interrogation showed the absence of any atrial arrhythmias.
Bipolar intracardiac electrocardiogram recordings from a coronary sinus and a LASSO catheter placed inside the left superior pulmonary vein. The first low amplitude and low frequency signal on LASSO 1-2 to 5-6 is the left atrial activation followed by sharp high frequency pulmonary vein potential recordings on L11-12 to L19-20. The earliest activation in the pulmonary vein is at L34 and L56 (arrow). Below, the epicardial view during the thoracoscopic ablation is shown. Notice the epicardially visible scar tissue from the previous ablation at the carina and at the anterior inferior left superior pulmonary vein in a location distal to the ostium of the veins. In contrast, the ablation line placed epicardially by a bipolar clamp under direct visual control by the surgeon is in an antral location proximal to the ostium of the pulmonary vein. LSPV, left superior pulmonary vein; RSPV, right superior pulmonary vein.
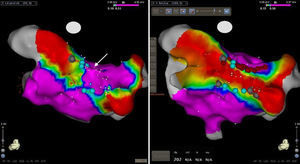
Posterior view of the bipolar left atrial voltage map following epicardial thoracoscopic ablation. Points with local bipolar voltage values of 0.5mV or greater are shown in purple, sites with low amplitude fragmented potential in blue, and those without visible local bipolar signals in gray. Notice the presence of scar or fragmented local activity along the epicardially placed inferior posterior and lateral part of the superior ablation lines. High amplitude normal potentials were observed on the septal part of the roof line on the endocardial surface (arrow). On the right side, the bipolar voltage remap is shown following endocardial “touch up” applications at the medial part of the roof line (red tags) and a scar with very low amplitude fragmented potentials along the inferior line. Notice the absence of local bipolar signals greater than 0.15mV inside the box lesion, suggesting entry block.
In addition to catheter ablation, new surgical ablation techniques and tools are emerging.1 Research in this area has been stimulated by the reported excellent long-term results after surgical Cox-Maze procedure.2 Recent advances in ablation techniques and thoracoscopic surgery have allowed surgeons to reproduce the classical cut-and-sew lesions set of the Cox-Maze procedure through a truly minimally invasive epicardial thoracoscopic approach. The preliminary results with these techniques were encouraging,3 with the additional advantages of simultaneous closure of the LA appendage. However, achieving and demonstrating PV entry and exit block and bidirectional conduction block along linear ablation lines remains a challenge. A recently introduced new technique to improve clinical results is the combination of a surgical and an electrophysiological approach. Our case illustrates the feasibility and advantages of using a three-dimensional mapping system during a simultaneous hybrid procedure in patients with persistent atrial fibrillation. In these patients, PV isolation is usually insufficient and a posterior box lesion is created. Testing bidirectional conduction block followed by endocardial completion in cases of incomplete multiple long linear lesions might be challenging with the use of fluoroscopy and surgical instruments as the only anatomical guide. We believe that visualization of the epicardial lines through mapping from the endocardial surface and the possibility of revisiting the tagged sites for ablation greatly facilitates the achievement of PV and posterior box isolation.
In our case, we report the presence of a visible epicardial scar on the left anterior PV ostium as a result of the first ablation. The scar was located inside the superior PV and ostium on the carina by the inferior vein. This example nicely illustrates the advantage of surgical PV isolation, when the level of the isolation is under direct visual control and is thus surely antral.
CONFLICTS OF INTERESTDr. La Meir has been consultant/advisor for Atricure and Estech. Dr. Moisés Rodríguez-Mañero is the holder of an unconditional grant from the European Society of Cardiology for post-residency training in electrophysiology.