Cardiogenic shock (CS) after myocardial infarction is associated with elevated mortality.1 There are few specific treatment options. Catecholamine administration may worsen tachycardia because decreased tissue perfusion may lead to reduced ventricular efficiency and increased oxygen consumption.2 Preliminary data indicate that ivabradine may offer a benefit in situations of severe tachycardia and shock, probably as a result of lower oxygen consumption and oxidative stress,2 although the hemodynamic effects of the drug in this context are unknown. For this reason, prior to administration of the drug in a clinical setting, we deemed it appropriate to study whether ivabradine administration may induce hemodynamic changes in a porcine model of CS after myocardial infarction.
Ten female large white pigs (mean weight, 32.8 [2.2]kg) were included. The animals were anesthetized with propofol and fentanyl and the anterior descending artery was occluded for 45minutes by inflation of an angioplasty balloon. To simulate CS after infarction, noradrenalin, dobutamine, and saline solution were administered until a postreperfusion heart rate (HR) of ≥ 90 bpm and a pulmonary wedge pressure > 18mmHg were achieved. Amiodarone was also administered at the same dose in both study groups to prevent ventricular fibrillation, which is a frequent occurrence in porcine models of acute ischemia. Hemodynamic parameters (blood pressure, HR, cardiac output, pulmonary artery pressure, pulmonary wedge pressure, and central venous pressure) were monitored with Swan-Ganz catheters inserted into the aorta via the carotid and jugular approach. After balloon deflation, each animal was stabilized for 15minutes prior to subsequent open-label randomization to the control group (n=5) or ivabradine group (n=5). Ivabradine was administered intravenously as a slow intravenous bolus at a dose of 0.3mg/kg and was diluted in distilled water at a concentration ≥ 12 mg/mL.3 The placebo group received the equivalent volume of saline solution. The aforementioned hemodynamic parameters were then measured at 15-minute intervals after infusion of drug/placebo. The study variables are expressed as mean±SD. The means were compared with the Student t test for independent data with a normal distribution and with the Fisher-Pitman test for independent variables with a nonparametric distribution.
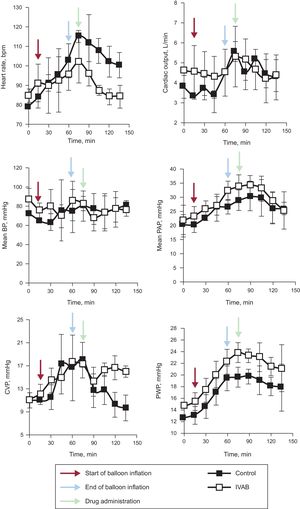
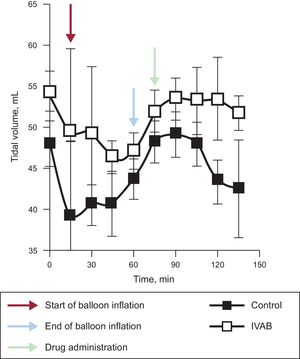
Ivabradine administration was associated with a significant decrease in HR (Figure 1; median [confidence interval] absolute reduction in HR at 15minutes, 21 [21 to 25] vs –1 [–5 to 0] bpm; P=.04), with no change in blood pressure, pulmonary artery pressure, or cardiac output. Tidal volume significantly increased in the ivabradine group (Figure 2; tidal volume at 15minutes, 63.7 [5.7] vs 43.7 [7.5]mL; P<.01). However, the decrease in HR was not accompanied by a reduction in pulmonary wedge pressure, and an increase in central venous pressure was observed compared with the control group (Figure 1). The numerical differences recorded in the 2 groups before administration of the study drug were not significant for any variables.
Change in hemodynamic parameters in ivabradine and control groups. Black arrows represent the start of coronary occlusion; blue arrows the end of occlusion; and red arrows the start of drug or placebo infusion. BP, blood pressure; bpm, beats per minute; CVP, central venous pressure; IVAB, ivabradine; PAP pulmonary artery pressure; PWP, pulmonary wedge pressure.
Although findings indicative of the efficacy and safety of ivabradine in acute heart failure after infarction have been reported,4,5 the hemodynamic impact of reducing HR in CS is not known. Our results are in agreement with those of Bakkehaug et al.6 in a porcine model of CS. The model used by those authors, however, was more invasive than ours—medial sternotomy was performed—and is thus less readily applicable in clinical practice. An additional consideration, at least as important as the type of model, is that the animals in that study were not randomized; rather, the animals were their own control. An effect of spontaneous improvement occurring after induction of ischemia and reperfusion of the infarction cannot therefore be ruled out.
In conclusion, ivabradine administered in a porcine model of CS induced by ischemia/reperfusion can reduce HR without significantly compromising cardiac output and can therefore increase tidal volume. However, this reduction in HR does not appear to reduce filling pressures. Before randomized clinical studies are conducted, we believe broader knowledge is required, in particular with a view to establishing whether this pharmacological strategy is of any value in reducing oxidative stress and myocardial damage in CS after myocardial infarction.
FUNDINGThis study received a grant from the Spanish Society of Cardiology project for Basic Research in Cardiology in 2015.
CONFLICTS OF INTERESTServier provided ivabradine in powder for intravenous administration free of charge.