INTRODUCTION
Dynamic left ventricular outflow tract obstruction (DLVOTO) is a common observation in hypertrophic cardiomyopathy. Nevertheless, it has also been reported under other circumstances such as dobutamine administration,1 postoperative period for mitral,2 or aortic3,4 valve surgery, or acute coronary syndromes.5-7 Dobutamine-induced DLVOTO was described by Pellikka1 in 1992 as a phenomenon that appears in 21% of dobutamine echocardiograms. Later, its appearance has been related to unexplained chest pain in patients with normal echocardiogram at rest,8 to angina in patients with normal coronary angiogram,9 and to exertional dyspnea in elderly patients.10 It has also been suggested that its appearance could be improved or eliminated with beta-blockers,11 although its clinical and prognostic significance has not been definitively established.
The fact that DLVOTO appears in some patients during dobutamine echocardiography suggests that it might also appear during exercise. However, the observation of this phenomenon during exercise has been reported in the literature only a few times,12-14 and thus the factors related to its appearance and its potential clinical significance have not been established. Exercise- or stress-induced dynamic obstruction that disappears upon rest could produce symptoms, with these symptoms hard to explain in hearts with little or no structural abnormalities. If this hypothesis is verified, it could identify a possible cause of angina or dyspnea in patients without evidence of cardiomyopathy or coronary disease, leading to therapy based on negative inotropic drugs. Hence, this study was undertaken with the following objectives:
1. To perform a prospective study on the possible appearance of DLVOTO during exercise.
2. To determine its incidence and extent, and the factors related to its appearance.
3. To analyze the clinical progress of patients with this condition.
PATIENTS AND METHODS
Patients
Two hundred and eleven consecutive patients referred for stress testing were studied; 134 of these patients met the following inclusion criteria:
1. Clinical symptoms of dyspnea or chest pain with an intermediate probability of coronary disease.
2. Possibility to perform stress echocardiography with adequate technical quality.
3. Completion of exercise until the submaximal heart rate was exceeded or presenting evidence of ischemia.
4. Sinus rhythm.
Patients with the following were excluded: a) known history of coronary disease (myocardial infarction or documented ischemia); b) overall or regional contractility alterations in the baseline tracing; c) moderate to severe valvular disease; d) left ventricular hypertrophy with no history of hypertension; and e) presence of dynamic subaortic obstruction to any degree in the baseline echocardiogram or family history of hypertrophic cardiomyopathy.
Stress Echocardiography
All patients were tested following the Bruce treadmill protocol after 4 h of fasting and discontinuation of anti-ischemic drugs for the previous 72 h. The test was considered valid if the submaximal heart rate was exceeded or evidence of myocardial ischemia appeared, with the latter defined as a horizontal or descending ST-segment depression greater than 0.1 mV at 80 milliseconds after the J point in the electrocardiogram.
The echocardiographic examination was performed using a VingMed ultrasound machine equipped with Super-VHS recording system and Pinnacle DV500 Plus system, using a 2.5-3.25 MHz probe. Baseline 2-dimensional M-mode echocardiography images were obtained by color and spectral Doppler ultrasound. Left ventricular outflow tract diameter was measured in the longitudinal plane during systole as the shorter distance between the anterior mitral valve and the interventricular septum. Immediately after exercise (60-120 s), echocardiographic images were acquired with the patient in lateral decubitus, starting with ventricular flow.
M-mode echocardiographic measurements of the left ventricle were taken in accordance with the recommendations of the American Society of Echocardiography (ASE),15 and left ventricular mass was calculated according to the modified Devereux formula16 for the ASE standards:
LVmass=0.80{1.04[IVSTd+PWTd+LVIDd]3LVIDd3}+0.6 g
where LV is the left ventricle; IVSTd, intraventricular septal thickness at end-diastole; PWT, posterior wall thickness at end-diastole; and LVIDd, left ventricular internal dimension at diastole.
Both the dimensions and the mass are expressed as indexed to body surface area.
Hypertrophy was considered to exist when the left ventricular mass index was above 125 g/m2, in accordance with studies identifying this as an effective cutoff to predict cardiovascular events.17,18
Left ventricular geometry was also assessed by calculating the relative wall thickness from the following formula:
RWT=IVSTd+PWTd/LVIDd
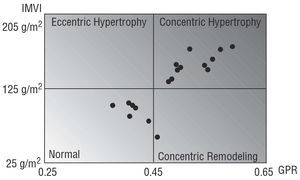
Based on a relative wall thickness >0.45 and the presence or absence of hypertrophy, patients were classified as having one of 4 types of ventricular geometry with prognostic significance:18,19 normal, concentric hypertrophy, eccentric hypertrophy, and concentric remodeling; the possible relation between type of ventricular geometry and appearance of exercise-induced DLVOTO was then analyzed.
Follow-up
Follow-up consisted of a medical history review or phone interview with the patients or their relatives to identify cardiovascular events such as death, myocardial infarction, unstable angina, heart failure, documented arrhythmia, or syncope.
Definition of Transient Dynamic Left Ventricular Outflow Tract Obstruction
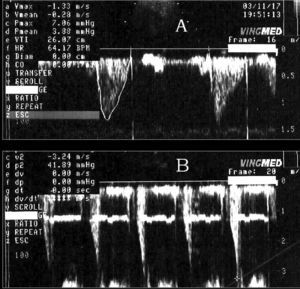
As established in other publications,4,20 DLVOTO was defined as the presence of systolic flow at a velocity equal to or greater than 2.5 m/s (equivalent to 25 mm Hg) and dagger-shaped late peaking in the left ventricular outflow tract or mid-ventricular region not present at baseline and disappearing after the recovery phase (Figure 1).
Figure 1. Continuous Doppler recordings of left ventricular outflow tract velocity, obtained at rest (A) and immediately post-exercise (B).
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation, and qualitative variables as percentages. The variables analyzed were compared in 2 groups according to the appearance of DLVOTO or not. The Chi-square test was used for qualitative variables, and one-way analysis of variance for continuous variables. Significance was set at a P-value <.05. Multivariate analysis was also performed using a multiple logistic regression model to identify independent predictive variables of the appearance of DLVOTO, with this model including those variables which reached a significance level below 0.1 in the univariate analysis. The odds ratio (OR) and 95% confidence intervals (CI) were calculated from the parameters estimated by the regression model. SPSS for Windows was used for the statistical analysis.
RESULTS
General and Clinical Data for the Patients
A total of 134 patients (59 women, 75 men) with a mean age of 58±9 years (range, 37 to 76 years) were included; 69.4% had hypertension; 35.8%, dyslipidemia; 9%, active smoker, and 24.6%, diabetes mellitus.
Symptoms were chest pain in 67.9% of the patients and exertional dyspnea in 32.1%; the mean functional class was 1.71±0.60.
Effort-Related Data
Post-exercise echocardiogram detected DLVOTO in 18 (13.4%) patients, with a gradient of 25 to 53 mm Hg (mean, 32.19±6.63 mm Hg), that disappeared in the following minutes during the recovery phase. These patients were considered group A, and the 116 remaining patients, group B.
A comparative analysis of the demographic and clinical variables (Table 1) showed that both groups were similar except in the functional class, which was higher in group A. The behavior of both groups during the exercise was similar (Table 2). A comparison of the echocardiographic variables (Table 3) showed that the patients of group A had greater wall thickness and smaller outflow tract and ventricular chamber diameters. In contrast, the ratio of septal to posterior wall thickness and the parameters of systolic and diastolic function were similar in both groups. In terms of ventricular geometry, group A was more likely to present concentric hypertrophy (61.1% vs 18.96%; P=.045), although some patients in this group had normal geometry (Figure 2).
Figure 2. Distribution of patients with dynamic left ventricular outflow tract obstruction, according to ventricular morphology. RWT indicates relative wall thickness; LVMI, left ventricular mass index.
The post-exercise echocardiogram showed a similar ejection fraction for both groups. No patients in group A presented ischemia data, versus 6.03% in group B who did. In group A, 5 (27.7%) patients had precordial oppression during exercise that was identified as their usual symptoms. No patient presented alterations in the electrocardiogram or a disproportionate elevation of blood pressure. In 4 patients, the myocardial scintigraphy was normal; the remaining case had a perfusion defect, but the coronary angiography was normal. In 1 patient, mitral regurgitation with exertion was detected, along with systolic anterior motion of the mitral valve. These 5 patients presented significantly higher exercise-induced gradients (42.65±10.5 mm Hg vs 28.15±2.37 mm Hg; P<.0001) than the rest of the group A patients with no symptoms during exercise.
In group B, 7 patients presented disturbances in regional contractility, with transient mitral regurgitation accompanied by inferoposterior hypokinesia in one of them.
Multivariate Analysis
A logistic regression model was constructed with the following variables: functional class, prior treatment, ventricular and outflow tract diameter, septal and posterior wall thickness, ratio of septal to posterior wall thickness, left ventricular hypertrophy, relative wall thickness, heart rate, and maximum outflow tract velocity at baseline. The only independent predictive factor of the appearance of DLVOTO during the post-exercise period was outflow tract diameter, measured in the baseline echocardiogram and adjusted to body surface area (P<.0001), with an OR of 0.092 (0.029-0.275) for 95% CI.
Usefulness of Outflow Tract Diameter as a Predictive Parameter of Exercise-Induced DLVOTO
Considering the left ventricular outflow tract 9.25 cm/m2 as a predictor of exercise-induced DLVOTO, this parameter presents a sensitivity of 94%, a specificity of 82% and a positive predictive value of 45.9%. In contrast, a value above 9.25 cm/m2 has a negative predictive value of 98.9%.
Clinical Follow-up Data
Data on clinical progress were obtained for 131 (97.7%) of the 134 patients, with a mean time of 369.9±133.5 days (range, 172-722 days). Among the 3 patients lost in follow-up, 1 belonged to group A and the other 2, group B.
No events were recorded in group A; 2 patients who had presented with angina and a perfusion defect on exercise scintigraphy underwent coronary angiography, with normal results in both cases.
In group B, 1 episode of unstable angina was reported 6 months after the initial study, which had been negative, and the coronary angiography showed a single 90% lesion in the posterior descending artery. Based on the initial study results, another 15 patients from this group underwent a scheduled coronary angiography; significant lesions (stenosis, 50% in the left coronary and 70% in the rest of the epicardial coronary vessels) were found in 7 cases.
The findings in patients who underwent coronary angiography and the respective additional non-invasive examinations are shown in Table 4, indicating that 5 patients in group B could be classified as carriers of cardiac syndrome X, since they presented exertional angina, electrocardiographic abnormalities suggestive of ischemia, and normal coronary angiography.
DISCUSSION
In this prospective series of patients with an increased prevalence of hypertension and clinical symptoms of chest pain or exertional dyspnea with normal systolic function, DLVOTO was induced by exercise in 13.4% of the cases. This incidence is higher than the 1.7% reported by Peteiro et al12 for a variety of potential reasons. First of all, the characteristics of the study population differed. In the Peteiro series all patients underwent stress echocardiography, whereas our study excluded patients with proven coronary disease or ventricular dysfunction, significant valve disease, or ventricular hypertrophy in the absence of hypertension or insufficient exercise. Secondly, ventricular flow velocities were measured immediately after exercise in our patients and not after analyzing regional contractility, which could have contributed to recording a larger number of gradients, given the tendency for the gradient to disappear in the minutes after exercise. In a recent PubMed search, we found only four other publications13,14,21,22 related to exercise-induced intraventricular gradient in patients without hypertrophic cardiomyopathy. Two of them describe patients who showed DLVOTO during dobutamine echocardiography but not during exercise although the low number of cases (15 in one14 and 10 in the other21) make it impossible to draw definitive conclusions. In the third publication, Meimoun et al22 present 2 patients with exertional dyspnea of no apparent cause who showed DLVOTO with SAM of the mitral valve and mitral regurgitation in the dobutamine echocardiography. In both cases DLVOTO together with dyspnea reappeared in the stress echocardiography. In the last paper, Cotrim et al13 describe a similar condition in a young man.
The univariate analysis identified hypertrophy, particularly the concentric type (relative wall thickness >0.45), as a predictor of exercise-induced DLVOTO. This is consistent with the findings of Harrison et al23 in 1991, in which some elderly patients with hypertension and severe concentric hypertrophy presented had an mid-ventricular obstr uction in the Doppler echocardiogram at rest. It seems paradoxical that the group with a higher incidence of hypertrophy secondary to hypertension had a lower incidence of coronary disease; this is attributable to the sample size and the higher frequency of other risk factors in the group without DLVOTO.
Asymmetric hypertrophy with septal predominance was not a predictive factor for obstruction in our series. This could be due to the low number of patients with this condition among our study population.
In the multivariate analysis, the only predictive factor for DLVOTO was left ventricular outflow tract diameter as measured in the baseline echocardiogram. We found that a value ≤9.25 mm/m2 can predict exercise-induced DLVOTO with a sensitivity of 94%, a specificity of 82% and a positive predictive value of only 45.9%. In contrast, a value above 9.25 mm/m2 has a negative predictive value of 98.9%. A simple measurement in the conventional resting echocardiogram could rule out the possible appearance of obstruction.
The clinical significance of DLVOTO in the absence of hypertrophic cardiomyopathy is still under debate in the literature. In our series, a linear relationship could not be established between the appearance of a pressure gradient and the patient's symptoms. This is the result of several factors. First of all, the stress tests were not performed to reproduce symptoms, but to find evidence of ischemia, since this was the reason why the patients consulted. Secondly, left ventricular flow was recorded immediately after rather than during exercise; hence, the extent of the gradient might have been underestimated because it disappears quickly during the recovery phase. In addition, the decubitus position in which the measurements were taken could also contribute to decreasing the gradient due to the preload increase involved in lying down.
The percentage of group A patients with angina-like symptoms during the stress test was not very high (27.7%), although the gradients were significantly higher in this subgroup (42.65±10.5 mm Hg vs 28.15±2.37 mm Hg; P<.0001) than in the rest of the group A patients.
The patients with DLVOTO showed no exercise-related electrocardiographic abnormalities, and therefore syndrome X24 can be ruled out as a cause of their symptoms. Conversely, group B had 5 patients (3.7% of all patients studied for angina or exertional dyspnea) who could be classified as carriers of this syndrome and had findings consistent with the description by Zouridakis et al,25 as the stress echocardiogram did not detect ischemia in syndrome X patients.
We did not observe any episode of transient apical ballooning syndrome,26-28 which some authors, such as Penas-Lado et al29 and Barriales Villa et al,30 consider may be secondary to the appearance of a transient dynamic intraventricular gradient in situations of severe adrenergic stimulation or hypovolemia. However, other authors28 have related it to vasospasm, microcirculation disturbances, or an unusual anatomy of the left anterior descending artery.31 Longer follow-up of our patients might indicate whether or not their capacity to present DLVOTO makes them more likely to develop transient apical ballooning.
Patients who presented exercise-induced DLVOTO in our series had a good prognosis at mid-term, as no events were observed after a mean follow-up of one year. This is consistent with the progress described by Barletta et al20 in patients with dobutamine-induced DLVOTO, but contrasts with the adverse prognosis described for DLVOTO appearing postoperatively32,33 or in acute coronary syndromes,5,34-36 in which the use of inotropic drugs or vasodilators can worsen the obstruction or hemodynamic deterioration.
Limitations
In this study, flow velocities and intraventricular gradient were measured immediately after exercise, rather than during maximal exercise. This is inevitable with treadmill testing, but may mean a lower gradient measurement, as the gradient tends to decrease only a few minutes after exercise is stopped. Moreover, the echocardiogram was recorded while the patient was lying down, making it easier to obtain the images, but possibly contributing to decreasing the gradient by increasing venous return and preload.
Another limitation is the absence of any relation between gradient induction and the reproduction of symptoms in some patients. Among other reasons, this may be because the exercise was done to detect evidence of ischemia or to exceed the submaximal heart rate, rather than to reproduce the symptoms. In order to analyze this relationship, it would be necessary to do the comparison with a control group of symptom-free subjects having demographic characteristics similar to those of the patients.
Clinical Implications
The data from this study suggest that in the absence of hypertrophic cardiomyopathy, DLVOTO can be induced by exercise in some patients with angina or exertional dyspnea and no evidence of ischemia, and this could be the cause of their symptoms. More studies are needed to confirm this finding and to determine the response of these patients to negative inotropic treatment. If confirmed, transient DLVOTO would have to be considered another cause of angina with normal coronary angiography.
See Editorial on Pages 1139-42
This study was funded by a basic and clinical research grant from the Sociedad Española de Cardiología (SEC, Spanish Society of Cardiology) 2002.
Correspondence: Dr. F. Cabrera Bueno.
Madame Bovary, 21, casa 14. 29620 Torremolinos. Málaga. España.
E-mail: fonendo@hotmail.es