Keywords
INTRODUCTION
Embolisms are a serious complication of infective endocarditis and occur in some 22%-55% of all episodes.1 Their consequences are often devastating and their appearance is understood as an ominous prognostic sign. This is particularly true of embolisms that occur in the central nervous system; these have been the most studied owing to their seriousness and frequency.2-4 The importance of infective endocarditis-associated embolisms in the liver, spleen and kidney is much less well known. To date, no studies have been made on the clinical context, microbiological status or echocardiographic conditions in which they appear, nor on their influence on the need for valve or extracardiac surgery, nor on their importance in terms of final prognosis. The data that are available have been gathered from patients who have undergone surgery or at autopsy, and mainly focus on the morphological characterization of endocarditis.
The aims of the present study were: a) to analyze the demographic, clinical, microbiological and echocardiographic characteristics of patients with endocarditis and associated embolisms in the liver, spleen or kidney, comparing them to those of patients in which no such embolisms developed, and b) to determine the impact of these embolisms on patient prognosis.
MATERIALS AND METHODS
Patient Groups
The patients recruited to this prospective study had all been admitted to one of five participating hospitals between 1996 and 2002 with a definitive or probable diagnosis of infective endocarditis (according to Duke University criteria).5 All those finally included had left endocarditis (no. episodes=338). Information was collected on all patients, including their age, sex, and place of residence, whether they had any predisposing heart condition, the presence of underlying disease, the causal agent, the clinical manifestations of their disease, and any changes in their analytical variables. Microbiological data (including the number of blood cultures performed), the type of organism involved, sensitivity and synergy data, serological information and echocardiographic findings (both transthoracic and transesophageal) were recorded for each episode. The antibiotic treatment prescribed was also recorded (including its duration), as were all data on clinical progress. In patients who underwent heart surgery, the indication for such treatment and the type of intervention were recorded. Finally, the release date or date and cause of death were noted.
Definition of Terms
The patients were divided into two groups: group I patients had embolisms in the liver, spleen or kidney, group II patients had no embolisms in these organs.
The definition of terms referring to clinical presentation, echocardiographic features and treatment have all been previously described.6
All embolisms were diagnosed by the physician attending each patient, based on clinical data (symptoms and signs) and complementary tests (computed tomography [CT], ultrasound, magnetic resonance and coronary angiography). All embolisms were accompanied by some symptom that prompted their confirmation by imaging techniques. The choice of imaging technique depended on the suspicions raised and the clinical status of each patient. In all but 3 episodes, ultrasound was used. An abdominal CT scan (simple and with contrast if the patient's condition so allowed) was performed to confirm the echocardiographic findings or as a complementary procedure when these were inconclusive.
Statistical Analysis
All data were analyzed using SPSS software for Windows (v. 11.5). Categorical variables were compared using the χ² or Fishers test. Quantitative variables were compared using the Student t test for independent samples. The results for quantitative variables were expressed as the mean ± standard deviation (SD). The relative risk (RR) values obtained in univariate analysis were adjusted by the corresponding logistic regression models. Significance was set at P<.05.
RESULTS
Three hundred and thirty eight episodes of left endocarditis were analyzed (216 involving native valves, 122 involving prosthetic valves). Forty one embolisms were recorded in the 34 group I episodes: 34 in the spleen, 5 in the kidney and 2 in the liver. In the remaining 304 (group II) episodes, the disease progressed with no development of embolisms in these organs. Some 97.1% of episodes in group I, and 92.8% in group II, met the Duke University criteria for a definitive diagnosis of endocarditis (P=.34). Post-release follow-up time varied between 0 d and 1745 d, with a mean of 105 d (group I, 0-991 d, mean 101 d; group II, 0-1745 days; mean, 108 d).
Clinical Characteristics
The male:female ratio in group I was 1.83:1; in group II this figure was 1.84:1 (P=.90) (Table 1). The mean ages of the 2 groups were 54±14.9 years (range, 27 years to 88 years) and 58.5±15.5 years (range, 12 years to 88 years) respectively (P=.11). Four patients (11.8%) in group I were intravenous (IV) drug addicts; group II had 10 (3.3%) such members (P=.04).
Of the total 41 embolisms recorded, 29 (70.7%) were present at the time of admission. The distribution of underlying heart disease was similar in both groups (Table 2). The most common symptom in both groups was fever (at admission and in the previous days or weeks).
Some signs and symptoms were more common in patients with embolisms, e.g., abdominal pain, splenomegaly, cutaneous stigmata of endocarditis and septic shock (Table 1). No patient presented with acute abdominal syndrome in group I; 4 group II patients (1.3%) showed this condition (P=1).
Imaging Diagnosis of Embolisms
The studied embolisms were diagnosed by abdominal ultrasound (in 30 out of 34 group I patients) or abdominal CT. Twenty seven group I patients (80%) had pathological conditions. Abdominal CT was performed in 23 of the same 34 patient-episodes (67.6%) and all were found to have pathological conditions. In ultrasound examinations, the most common pathology found was infarction; this was seen in all patients but 6, 2 of whom had a hepatic abscess and 4 of whom had a splenic abscess. Computed tomography tests detected one further infarction and 1 splenic abscess in 2 patients for whom the ultrasound results were normal.
Microbiology
The causal agent was isolated in 100% of cases in group I and in 84.5% of cases in group II (P=.008). Identifications were made by blood culture in all group I patients except for one; the spleen and affected valve of this patient were positive for Gram positive bacilli. In group II 47 episodes (15.5%) showed negative cultures. The most common pathogen in group I episodes was S aureus (26.5%), whereas in group II the most common was S viridans (17.1%). Table 3 shows the distribution of the pathogens detected.
Valve Lesions
Vegetations were seen more often in the transesophageal echocardiograms of group I patients. However, the detection of vegetations was comparable in the 2 groups with TTE. Those of group I patients were larger (mean diameter 16.51±8.12 mm compared to 13.03±6.94 mm in group II patients; P=.01). The morphological characteristics of these vegetations, the presence of periannular complications, and the frequency of valve failure were similar (Table 4).
The sites of infection were similar in both groups, with native mitral valves the most frequently affected. Seven episodes of mitroaortic endocarditis were recorded among group I patients, and 72 among group II patients. A total of 122 episodes of prosthesis-associated endocarditis were also recorded (12 in group I, 110 in group II).
The existence of embolisms in locations other than the liver, spleen or kidney was more common among group I patients (18 episodes [52.9%] compared to 61 episodes [20.1%] in group II; P<.001). The majority of these embolisms (17 out of 18 [group I] and 60 out of 61 [group II]) were located in the central nervous system, and were present at the time of admission in 32.4% of episodes in group I and 15.1% in group II (P=.01).
Clinical Course
The length of hospital stay was slightly but not significantly longer for group I patients (Table 5). During the course of their illness, group I patients showed signs of persistent infection, septic shock, renal failure, and heart failure more commonly than did group II patients (Table 5).
Seventeen patients of group I (50%) and 164 of group II (53.9%) required heart surgery (P=.66); this was urgently required by eight group I patients (23.5%) and 66 group II patients (22.4%) (P=.71) The most common indication for surgery was heart failure, followed by signs of persistent infection. The indications for surgery were multiple in many patients. The presence of embolism was considered an indication for surgery in eight group I patients (47.1% of those who underwent surgery from this group); surgery was required in 14 group II patients who had embolisms in organs other than the liver, spleen or kidney (8.73%) (P<.001; Table 5).
Only 3 group I patients had embolisms that required specific surgery; a kidney punch biopsy was performed in one and splenectomy in 2.
Fourteen group I patients (41.2%) and 93 group II patients (30.6%) died (P=.20). Nine of the group I patients that died had exclusively splenic embolisms. The embolism of one patient was in fact an abscess caused by S aureus, but the patient's poor condition (septic shock) contraindicated surgery. Two of the patients with renal embolisms died (although the spleen was affected in both), as did both patients with a hepatic embolism (the spleen of one of these patients was also affected). Although the differences between the causes of death were not significant (P=.5), the most common in both patient groups was septic shock. No significant differences were seen in the mortality of patients who underwent or did not undergo surgery (Table 5).
DISCUSSION
The spleen is a particularly easy target for embolism damage owing to the characteristics of its anatomy and blood circulation. The occlusion of the splenic circulation, even by a sterile embolism, can lead to the formation of an abscess. In endocarditis, the presence of pathogens in embolic fragments is sufficient to give rise to a splenic abscess.7 The involvement of the kidney in endocarditis can be caused by 3 mechanisms: focal or diffuse glomerulonephritis through the deposit of immunocomplexes, renal failure, and renal abscess. The most common form of kidney involvement is embolic renal infarction.8 The liver may also be a target of hematogenous seeding via the arteries. However, the most common cause of hepatic abscesses are related to biliary or colonic disease, gastric or duodenal surgery, local trauma and pancreatitis.9
The frequency of appearance of embolisms in the spleen, kidney or liver has changed with the availability of antibiotics and the development of imaging techniques. In the pre-antibiotic era, the spleen was affected in 30%-67% of patients with endocarditis, whereas these figures now vary between 31% and 44%,10 with the most recent studies reporting values as low as 4.8%-35%. These latter data come from studies with different designs, including some in which patients were treated surgically,11 retrospective clinical studies,12,13 and clinical studies whose aim was to identify embolisms.14,15
In the present work the incidence of embolisms in the spleen, kidney and/or liver was 10% (10% in the spleen, 1.4% in the kidney, and 0.5% in the liver). These results are very similar to those reported by Millaire et al,15 even though these authors systematically searched for embolisms (symptomatic and non-symptomatic).
From an epidemiological point of view, the percentage of IV drug users was much lower than that seen in older studies, in which they made up to 68% of the population.11 No significant differences were seen between the 2 present groups with respect to age distribution, although a recent study reported younger age to be independently associated with larger embolisms.16
Clinical Presentation
One of the main characteristics of embolisms is their silent nature; their true frequency may therefore be routinely underestimated.7,17 This is probably why embolisms are found more commonly during autopsies than in live patients. In the present study, the most common clinical presentation of kidney or spleen failure was abdominal pain, although fever, pleural pain, back pain running into the left shoulder and splenomegaly-related symptoms were also reorded.18
In endocarditis, the majority of embolisms form before corrective antibiotic treatment begins, and their manifestations contribute to the presentation of the disease. The risk of new embolisms occurring is notably reduced after 2 weeks of antibiotic treatement.6 Some authors have highlighted the importance of early clinical suspicions in the reduction of complications.19 In the present study, 9 new embolisms appeared--3 splenic and 1 renal in the first week of treatment, 4 splenic and 1 renal in the second week. Beyond this point, new embolisms were rare: 2 new splenic embolisms were detected in the third week and 1 more in the sixth week. Frequently, embolisms that form after treatment has begun are manifested along with signs of persistent infection (due to the spread of the latter). Some 55.9% of group I patients showed such signs compared to 32.6% of group II patients (P=.007).
Microbiology
The causal pathogen of the episode of endocarditis was identified in all patients who suffered embolisms: in all except one patient identifications were made from blood cultures. S aureus was more commonly the cause in group I than in group II patients. This difference was maintained even when IV drug users were excluded, despite the fact that this very bacterium was the most common causal agent in these patients (8 [57.1%] of the 14 patients). The association between embolisms and certain types of pathogen has been reported; some studies have shown fungi and S aureus increase the risk of developing an embolism,6,20 especially if associated with vegetations >10 mm in diameter.6
Identification of Embolisms
As well as using clinical data, embolisms were diagnosed using abdominal CT, abdominal ultrasound or a combination of these techniques. The diagnostic performance of both was similar. Before CT became available, the imaging techniques used for diagnosing embolisms were ultrasonography and isotopic markers. Nowadays, CT is considered the technique of choice owing to its greater sensitivity and the absence of artifacts in the images produced. Air in the abdomen is the main problem when using ultrasound. However, some authors prefer this technique for the follow-up of lesions since it is completely innocuous (from the point of view of radiation damage) and is non-invasive. Techniques involving radioisotopes are now obsolete. Echocardiography can differentiate between recent and older infarctions since, during the evolution of these lesions, the affected areas become more nodular, fibrotic and echogenic.21
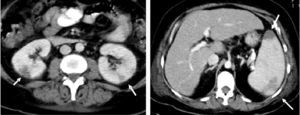
In CT, the classic image of a splenic infarction is a triangular area with its base in the periphery; its outline is well define d but hypointense, and it is not made more obvious by the use of contrast. However, it is more common to find diffuse, hypointense and poorly-outlined areas22 (Figure). With ultrasound, the classic image involves a hypoechoic but well defined region. The presence of a blood flow (as determined by the color Doppler technique) does not exclude a diagnosis of splenic infarction since the thrombus or embolism may have become smooth.21 In general, and independently of the technique used, splenic infarctions and abscesses are accompanied by splenomegaly.
Figure 1. Abdominal computed tomography images of a patient with endocarditis caused by Staphylococcus aureus. Left: note the 2 splenic infarctions (arrows); Right: bilateral renal infarctions (arrows).
Liver abscesses can take on the appearance of cysts, although they can usually be differentiated from these. Abscesses usually have fluid centers and thicker, more irregular and more evident walls than do simple cysts. Though in CT images the attenuation of the content of an abscess can appear similar to that of water, it is usually more dense. In ultrasound images, a layer of necrotic detritus can be seen inside abscesses. Cysts may also show hypoechoic regions; these are attributed to the presence of cholesterol crystals left over from old, intracyst hemorrhages. Hepatic abscesses are radiologically indistinguishable from necrotic tumors, either by CT, ultrasound or magnetic resonance techniques. In such doubtful cases, the clinical context of the patient should be considered in order for a diagnosis to be ventured.23
Valve Lesions and Echocardiographic Findings
Vegetations were more often found by TEE in group I than in group II patients; in both groups TEE was superior to TTE in the detection of vegetations. Reynolds et al24 recently showed (in a more favorable scenario involving patients with their native valves and good acoustic windows) that, despite technological advances in TTE, the sensitivity of TEE is still greater. In the present study, the diameter of the vegetations was greater in the group I patients. The association between vegetation size and the appearance of embolisms has been the object of several retrospective studies.17,25,26 In a meta-analysis27 and in a recent prospective study with a large number of patients, the association appeared to be clear.6
Clinical Course
The frequency of surgical treatment was greater among group I patients. No recommendations have been established, however, with respect to whether such embolisms are an indication for surgery. In our opinion, systemic embolisms do not necessarily mean a patient requires surgery, although they are factors that support such a decision. With respect to endocarditis, the Clinical Practice Guides of the Sociedad Española de Cardiología indicate that the existence of repeat embolisms along with persistent images of vegetations could mean surgery is advisable.28 Some authors even suggest that splenic embolisms should be systematically searched for in order to support any decision made.14 Bearing in mind that the majority of such episodes occur before antibiotic treatment begins, and that once such treatment has begun the chances of a new embolism appearing are drastically reduced, embolisms do acquire weight as an indication for surgery when episodes are multiple (despite antibiotic treatment) or when patients have large vegetations.29 In the present study, the mean diameter of the vegetations of the group I patients who underwent surgery was 20.60±7.1 2 mm, whereas in those who did not receive such treatment they measured 11.87±6.68 mm (P=.001). In general, the decision to operate in these patients--as in those who had no embolisms--was based on classic clinical indications (heart failure, persistent signs of infection); surgery is known to improve patient prognosis in such cases. A multivariate analysis was performed to establish the determinants for surgery among our patients, and it was found that liver, spleen or kidney embolisms were not independently associated with the need for valve surgery.
The need for extracardiac surgery was exceptional. In our experience, the course of endocarditis is rarely complicated severely by the presence of embolisms. Small infarctions can be treated in a conservative fashion,11 and it is quite normal for them to progress favorably, the associated pain gradually disappearing. When large, splenic or renal infarctions can develop into abscesses which can (rarely) cause the spleen to rupture or lead to kidney hemorrhaging (through the rupture of a mycotic aneurysm).30,31 Such events occur late in the course of illness. Splenectomy is only necessary when the loss of parenchymatous structure is extensive; such a development could provide a focus for sepsis and the organ could rupture.11 In such cases a sequential approach is recommended involving a laparoscopic splenectomy followed by valve replacement.32 Image-guided drainage of the abscess is also possible, but this is not effective when there are multiple lesions and there is a risk of hemorrhage.33
In the present study, the mortality of the group I patients was slightly greater, but not significantly so (41.2% compared to 30.6% for group II patients; P=.2). Table 6 shows the differences between patients that died and those that survived. Multivariate analysis showed that the presence of liver, spleen or kidney embolisms was not independently associated with greater mortality.
Associated morbidity was, however, greater in the patients with the studied embolisms. During the course of their illness they were more likely to develop embolisms at other sites (usually the central nervous system), to have signs of persistent infection, and to suffer septic shock or renal failure. These complications could be avoided, or at least anticipated, if the conditions associated with the appearance of liver, spleen or kidney embolisms were known. The following characteristics at the time of admission were independently associated with the presence of such embolisms: abdominal presentation (adjusted OR, 8.51), large vegetation(s) in the first TEE (adjusted OR, 1.10), and the presence of S aureus (adjusted OR, 2.68) or enterococci (adjusted OR, 3.88) (Table 7).
Limitations of the Study
This study has two main limitations. The first is the lack of anatamo-pathological correlations (due to the exceptional need for splenectomy or nephrectomy and the small number of necropsies performed on deceased patients); this lack of anatomical evidence impeded the corroboration of microbiological and radiological findings. The second is the size of group I; this reduced the capacity of the statistical tests to detect significant differences, especially in the area of prognostic features. Assembling a larger group of patients with the same characteristics (i.e., with clinically evident embolisms [and in a prospective study]) would be difficult, however, given the low incidence of these complications.
CONCLUSIONS
Liver, spleen and kidney embolisms are seen in about 10% of patients with endocarditis. The risk of developing these complications increases when the causal agent of the disease is S aureus or enterococcus, as vegetations become larger, and when patients present with abdominal symptoms. They are associated with embolisms in other organs, mainly the central nervous system. Vegetations are more commonly detected by TEE when large and when the studied embolisms are not present. No correlation was found between the presence of embolisms and the need for surgery or risk of death.
Correspondence: Dr. I. Vilacosta.
Servicio de Cardiología. Instituto Cardiovascular.
Hospital Clínico Universitario San Carlos.
Profesor Martín Lagos, s/n. 28040 Madrid. España.
E-mail: ivilac@medynet.com