In recent years, clinical research aimed at determining the effects of antihypertensive strategies—beyond simply reducing and controlling blood pressure—has intensified substantially. In fact, dual renin-angiotensin-aldosterone system (RAAS) blockade that simultaneously interferes with angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists or direct renin inhibitors (aliskiren) has focused these strategies on patients at high cardiometabolic risk.1
The possibility that dual RAAS blockade might yield better results than the use of each drug on its own is an attractive hypothesis. However, the ONTARGET (The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) study found that dual blockade with angiotensin II receptor antagonists (telmisartan) and an angiotensin converting enzyme inhibitor (ramipril) offered no additional benefit (vs monotherapy) in reducing cardiovascular morbidity and mortality in patients at high cardiovascular risk and did, in fact, increase the incidence of adverse effects.2
More recently, the ALTITUDE (Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints) study was interrupted prematurely because dual therapy was not showing clinical benefits in patients with diabetes being treated with aliskiren and angiotensin converting enzyme inhibitors or angiotensin II receptor antagonists, whereas renal complications, hyperkalemia, hypotension and stroke, among other adverse effects, increased.3
The present study evaluated the risks of dual RAAS blockade in patients with diabetes mellitus through a meta-analysis of randomized, controlled clinical trials. For this purpose, we reviewed PubMed up to November 2012 and consulted the Spanish agency for medicines and healthcare products (Agencia Española de Medicamentos y Productos Sanitarios) (www.aemps.gob.es), the European Medicines Agency (www.ema.europa.eu) and the US Food and Drug Administration (www.fda.gov) online. The following search terms were used: renin inhibitor, aliskiren, angiotensin receptor block*, losartan, irbesartan, valsartan, olmesartan, candesartan, eprosartan, telmisartan, angiotensin-converting enzyme inhibit*, captopril, enalapril, lisinopril, perindopril, ramipril, fosinopril, trandolapril, temocapril, imidapril combined with diabetes, diabetic*, and randomized controlled trial [publication type]. We included studies of patients with diabetes using the (dual) combination of RAAS blockers in the intervention group vs any RAAS blocker in monotherapy, and presenting data on hyperkalemia, hypotension, kidney damage and/or death from any cause. We used the definitions proposed in each of the studies identified.
We calculated relative risks in combination with their 95% confidence intervals by using fixed effects and random effects models. We determined the degree of heterogeneity in each study and among studies using the Cochran Q statistic and I2 index. Statistical analysis was with STATA 12® (StataCorp LP; Collage Station, Texas, United States).
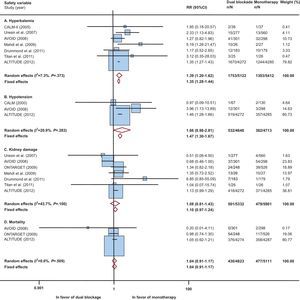
We selected 9 studies with 11 543 patients with diabetes (Table) (references in the supplementary material). The meta-analysis results showed that patients with diabetes receiving dual RAAS blockade had a higher risk (vs monotherapy) of hyperkalemia, hypotension and kidney damage, with no reduction in overall mortality. We found no significant heterogeneity. In patients with hypotension and kidney damage, increased risk was statistically significant (or nearly so) in only 1 of the models (Figure). In general, and except for kidney damage, eliminating the most influential study (ALTITUDE) did not substantially alter the results of the analyses (supplementary material).
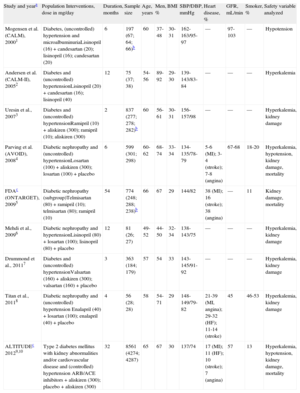
Studies Selected and Baseline Characteristics of Patients With Diabetes
| Study and yeara | Population Interventions, dose in mg/day | Duration, months | Sample size | Age, years | Men, % | BMI | SBP/DBP, mmHg | Heart disease, % | GFR, mL/min | Smoker, % | Safety variable analyzed |
| Mogensen et al. (CALM), 20001 | Diabetes, (uncontrolled) hypertension and microalbuminuriaLisinopril (16) + candesartan (20); lisinopril (16); candesartan (20) | 6 | 197 (67; 64; 66)b | 60 | 37-48 | 30-31 | 162-163/95-97 | — | 97-103 | — | Hypotension |
| Andersen et al. (CALM-II), 20052 | Diabetes and (uncontrolled) hypertensionLisinopril (20) + candesartan (16); lisinopril (40) | 12 | 75 (37; 38) | 54-56 | 89-92 | 29-30 | 139-143/83-84 | — | — | — | Hyperkalemia |
| Uresin et al., 20073 | Diabetes and (uncontrolled) hypertensionRamipril (10) + aliskiren (300); ramipril (10); aliskiren (300) | 2 | 837 (277; 278; 282)b | 60 | 56-61 | 30-31 | 156-157/98 | — | — | — | Hyperkalemia, kidney damage |
| Parving et al. (AVOID), 20084 | Diabetic nephropathy and (uncontrolled) hypertensionLosartan (100) + aliskiren (300); losartan (100) + placebo | 6 | 599 (301; 298) | 60-62 | 68-74 | 33-34 | 134-135/78-79 | 5-6 (MI); 3-4 (stroke); 7-8 (angina) | 67-68 | 18-20 | Hyperkalemia, hypotension, kidney damage, mortality |
| FDAc (ONTARGET), 20095 | Diabetic nephropathy (subgroup)Telmisartan (80) + ramipril (10); telmisartan (80); ramipril (10) | 54 | 774 (248; 288; 238)b | 66 | 67 | 29 | 144/82 | 38 (MI); 16 (stroke); 38 (angina) | — | 11 | Kidney damage, mortality |
| Mehdi et al., 20096 | Diabetic nephropathy and hypertensionLisinopril (80) + losartan (100); lisinopril (80) + placebo | 12 | 81 (26; 27) | 49-52 | 44-50 | 32-34 | 138-143/75 | — | — | — | Hyperkalemia, kidney damage |
| Drummond et al., 20117 | Diabetes and (uncontrolled) hypertensionValsartan (160) + aliskiren (300); valsartan (160) + placebo | 3 | 363 (184; 179) | 57 | 54 | 33 | 143-145/91-92 | — | — | — | Hyperkalemia, kidney damage |
| Titan et al., 20118 | Diabetic nephropathy and (uncontrolled) hypertension Enalapril (40) + losartan (100); enalapril (40) + placebo | 4 | 56 (28; 28) | 58 | 54-71 | 29 | 148-149/79-82 | 21-39 (MI, angina); 29-32 (HF); 11-14 (stroke) | 45 | 46-53 | Hyperkalemia, kidney damage |
| ALTITUDEc 20129,10 | Type 2 diabetes mellitus with kidney abnormalities and/or cardiovascular disease and (controlled) hypertension ARB/ACE inhibitors + aliskiren (300); placebo + aliskiren (300) | 32 | 8561 (4274; 4287) | 65 | 67 | 30 | 137/74 | 17 (MI); 11 (HF); 10 (stroke); 7 (angina) | 57 | 13 | Hyperkalemia, hypotension, kidney damage, mortality |
ACE, angiotensin-converting enzyme; ALTITUDE, Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints; ARB, angiotensin receptor blocker; AVOID, Air Verses Oxygen In myocarDial infarction study; CALM, Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria study; DBP, diastolic blood pressure; FDA, Food and Drug Administration; GFR, (mean) glomerular filtration rate; HF, heart failure; MI, myocardial infarction; ONTARGET, ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial; SBP, systolic blood pressure.
Risks of dual renin-angiotensin-aldosterone system blockade in patients with diabetes. 95%CI, 95% confidence interval; ALTITUDE, Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints; AVOID, Air Verses Oxygen In myocarDial infarction study; CALM, Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria study; ONTARGET, ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial; RR, relative risk.
Cardiovascular and kidney disease are the principle causes of morbidity and mortality in patients with diabetes. Some clinical practice guidelines4 recommend more aggressive antihypertensive treatments (such as dual RAAS blockade) to achieve therapeutic objectives of blood pressure<130/80 mmHg, despite the controversy over their additional clinical benefit. Although methodological and population-based differences exist, the ALTITUDE results confirmed ONTARGET predictions, which had already shown that the balance between the benefits and risks of RAAS inhibitor combinations was negative, mainly because of the increased incidence of severe adverse effects. Indeed, technical data on these products already recommended against concomitant use of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists inhibitors in controlled hypertense patients. Furthermore, in individually defined cases, these data suggest that renal function and blood potassium concentrations should be closely controlled.5 Recently, because of ALTITUDE results, intense or dual RAAS blockade with aliskiren and angiotensin converting enzyme/angiotensin II receptor antagonists inhibitors in patients with diabetes or moderate-severe kidney failure has been contraindicated.3
Our present results coincide with those of earlier studies in more heterogeneous populations.6 However, our analysis is limited by the quality of the information used and the scarcity of studies in the population with diabetes—in some cases, with a minimal sample size. This limitation prevents us from narrowing down treatments beyond 2 broad-ranging groups (dual RAAS blockade vs monotherapy).
.