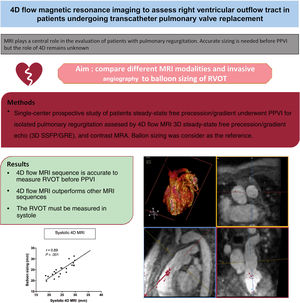
Magnetic resonance imaging (MRI) including 4D flow is used before percutaneous pulmonary valve implantation (PPVI). As PPVI is limited by the size of the right ventricular outflow tract (RVOT), accurate sizing is needed to plan the intervention. The aim of this study was to compare different MRI modalities and invasive angiography to balloon sizing of RVOT.
MethodsSingle-center prospective study of patients who underwent PPVI for isolated pulmonary regurgitation assessed by 4D flow MRI, 3D steady-state free precession/gradient echo (3D SSFP/GRE) and contrast magnetic resonance angiography. Balloon sizing was considered as the reference.
ResultsA total of 23 adults were included (mean age, 38.4±12.5 years). Eighteen patients underwent successful primary PPVI. The average of the narrowest RVOT diameter was 25.4±4.3 mm by balloon sizing. Compared to balloon sizing, RVOT diameters were better correlated when estimated by systolic 4D flow MRI (r=0.89, P<.001) than by diastolic 4D flow MRI (r=0.71, P <.001), 3D contrast magnetic resonance angiography (r=0.73; P <.001) and 3D SSFP/GRE (r=0.50; P=.04) and was not significantly correlated when estimated by 2D in diastole and systole. The mean difference between systolic 4D flow MRI and balloon sizing was 0.2 mm (95%CI, –3.5 to 3.9 mm), whereas it was wider with other techniques.
ConclusionsBeyond the quantification of pulmonary valve regurgitation, 4D flow allows accurate estimation of RVOT diameters, especially in systole, which is fundamental before planning PPVI.
Keywords
Right ventricular outflow tract (RVOT) dysfunction is a major concern in adults with congenital heart disease (CHD). After 40 years, the cumulative incidence of pulmonary valve replacement (PVR) in patients with tetralogy of Fallot is around 50%.1
Magnetic resonance imaging (MRI) is the cornerstone of the decision to perform PVR in patients with (RVOT) dysfunction, as highlighted by guidelines.2,3 Indeed, enlargement and function as well as the severity of pulmonary regurgitation are accurately assessed by MRI.4
Since 2000, percutaneous pulmonary valve implantation (PPVI) has emerged as an alternative to surgical replacement.5,6 Nevertheless, the suitability of the devices is still a major issue. In one study, nearly half of the patients presenting for catheterization did not undergo PPVI, mainly because of prohibitively large size of the RVOT, especially when there is no RVOT obstruction.7 Thus, accurate assessment of the morphology and size of the RVOT by noninvasive measurements is necessary to improve the selection of patients who can benefit from a PPVI and those who will directly undergo surgery.
4D flow as a time-resolved volumetric phase-contrast imaging with 3 directional velocity encoding is becoming part of the comprehensive evaluation of CHD in MRI.8–10 As well as flow and hemodynamic information,11 4D flow provides time-resolved volumetric anatomical information that may be suitable for accurate sizing of the RVOT throughout the cardiac cycle without adding scan time.
The aim of this study was to compare the measurements of native RVOT between different MRI imaging sequences, including 4D flow, and catheterization findings.
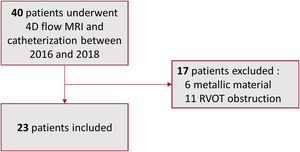
METHODSThis single-center study included 23 consecutive patients over a 24-month period who underwent catheterization for PPVI between 2016 and 2018. All patients underwent MRI prior to the PPVI procedure (figure 1).
Inclusion criteria were consisted of adult patients (< 18 years) with pulmonary regurgitation related to CHD assessed by MRI and requiring PPVI according to the standard of care.
Exclusion criteria comprised patients with prior metal material in the pulmonary tree, cardiac pacemaker, or defibrillator. Patients with significant residual RVOT obstruction assessed by transthoracic echocardiography were also excluded. Significant obstruction was defined as a maximal right ventricular to pulmonary artery gradient above 50 mmHg or a right ventricular systolic pressure above half of the systemic pressure. The institutional review board approved this research protocol (CPP n° 2014-11-06) and individual consent and a signature was obtained for each patient.
All MRI measurements were blinded to catheterization measurements and performed retrospectively by the same operator (G. Soulat). MRI measurements did not guide the PPVI vs surgical PVR referral. Since 2019, our center has used 4D flow MRI to guide referral to catheterization or surgery. Balloon sizing during PPVI was used as the reference. In our routine protocol, no patient underwent computed tomography (CT).
Catheterization protocol and data analysisPPVI was performed under general anesthesia and under monoplane fluoroscopic guidance (Siemens Medical Solutions, Germany) using a standardized approach.12 RVOT angiography was performed in 2 orthogonal plans to assess and measure the proposed site for device implantation. Invasive pulmonary angiography (IPA) measurements were performed in systole and diastole. Sizing was then performed with a sizing balloon using a compliant 30 mm Tyshak balloon (NuMed, United States). Measurements were performed in 2 orthogonal plans and the minimal diameter of the RVOT was measured and averaged ([diameter 1 + orthogonal diameter 2]/2) for each method (diastolic IPA, systolic IPA, and sizing balloon). All catheterization measurements were performed after sizing to a known distance in the RVOT (multitrack angiocatheter or Tyshak balloon).
PPVI was performed using various methods of valve implantation with the Melody valve (Medtronic, United States) or more recently the SAPIEN valve (Edwards Lifesciences, United States).6,13,14
Magnetic resonance imaging protocol and data analysisMRI examinations were performed on a 1.5 or 3 Tesla system (Signa HDxT, Artist, Discovery MR 750w, or Architect systems, GE Healthcare, United States) with an 8- or 32-channel cardiac phased array surface coil. Precontrast cine steady-state free precession (SSFP) sequences were acquired in axial, long-, and short-axis views during breath-hold to cover the entire RV, as recommended.15 Diastolic gated 3D SSFP at 1.5 T (scan parameters: spatial resolution=2.1 x 2.1 x 3.6 mm, flip angle 65°, TR 3.6ms, TE 1.6ms) or 3D Gradient echo at 3T (scan parameters: spatial resolution=2.2 x 2.2 x 2.8 mm, flip angle 20°, TR 3.1ms, TE 1.3ms) and three 2D cine SSFP planes orthogonal to the RVOT (scan parameters: spatial resolution 7.0 x 1.8 x 2.1 flip angle 50°, TR 3.1ms, TE 1.32) orthogonal to the minimal areas and obtained in a single breath-hold were successively acquired. Finally, an ungated 3D contrast magnetic resonance angiography (MRA) at both 1.5 and 3T (scan parameters: spatial resolution=0.7-0.8 x 1.9-2.2 x 2.4-3.0 mm, flip angle 25-30°, TR 3.2-3.9ms, TE 1.1-1.3 ms) of the pulmonary tree were also acquired in a single breath-hold (figure 2). Injection of a 0.20 mmol/kg gadolinium contrast agent was usually performed (gadobenate dimeglumine or gadoterate meglumine) during the MRA acquisition.
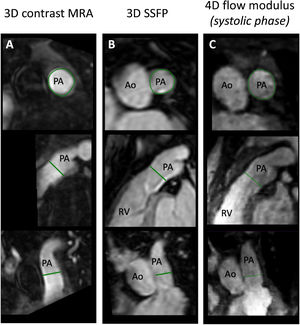
Example of right ventricular outflow tract measurement (RVOT). Three perpendicular planes of the RVOT at the same location in the same patient using contrast magnetic resonance angiography (MRA) (A); 3D steady-state free precession (SSFP) (B); and 4D flow magnitude at the systolic phase (C). Diameter measurements were taken on the plane perpendicular to the centerline (at the top) and averaged. Ao, aorta; PA, pulmonary artery; RV, right ventricle.
Video 1 of the supplementary data illustrates the interest of 4D (3D volume plus time) methods in the analysis of these complex geometric structures moving and orienting themselves differently over time.
A close to 8-minute electrocardiogram (ECG)-gated 4D-flow MRI acquisition was performed immediately after the gadolinium injection in a sagittal volume orientation covering the entire cardiac volume. The pulse sequence consisted of retrospective-ECG-gated gradient echo sequence with 3-directional velocity encoding (encoding velocity=250 to 450cm. sec-1 in all directions to avoid velocity aliasing in the RVOT).
Scan parameters were as follows: average spatial resolution=2.0 x 2.4 x 1.8 mm, flip angle 15°, TR=4.2 to 5ms, TE=1.60ms, bandwidth 62kHz, views per segment=2. Either the research or product sequence were use as available at the time of the initial scan. 4D flow postprocessing was carried out using in-house software (Lattido, Universidad Favaloro–CONICET, Buenos Aires, Argentina & Université de Paris, France). Measurements were taken using only the magnitude imaging, perpendicular to the centerline of the vessel using multiplanar reformatting in end diastole and end systole.
For all 3D techniques, measurements were taken perpendicular to the centerline at the narrowest part of the RVOT, from the pulmonary annulus to pulmonary bifurcation. The maximum and minimal diameters were averaged as illustrated in figure 2.
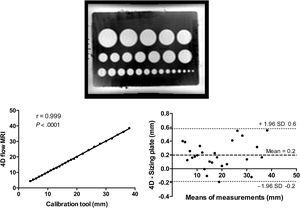
Phantom studyTo our knowledge, since 4D flow was not validated for length measurement, we performed a phantom study to test the accuracy of diameter and length measurements. A reference sizing table for estimating atrial septal occlusion devices (with calibrated diameters) was placed in a water tank containing a gadolinium solution to serve as a stationary phantom to test the clinical sequences (figure 2).
ReproducibilityMRI measurements were taken by G. Soulat with 7 years of experience in CHD MRI. To assess interobserver variability,16 a 4D flow dataset was analyzed by a second operator (Y. Alattar) with 3 years of experience and who was blinded to the previous results.
Statistical analysisBaseline characteristics are provided as mean±standard deviation (SD) or median [inter quartile range], as appropriate. Univariate correlations between each modality of measurements were reported using Pearson correlation coefficients, if the normality test was passed with Shapiro-Wilk test and represented on a Bland-Altman plot. To test the interobserver reproducibility of the measurements with 4D flow, the intraclass correlation coefficient (ICC) was calculated. A P <.05 was used to indicate statistical significance. An analysis was carried out using GraphPad Prism version 6 (GraphPad Software, United States).
RESULTSPatient characteristics are summarized in table 1. CHD was a previous repaired tetralogy of Fallot in all patients but 3, with a mean age of 38.4±12.5 years old. All patients had a native RVOT but 1 homograft.
Patient characteristics
| N=23 | |
|---|---|
| Age, y | 38.4 [18-57] |
| Male, sex | 11 (47.8) |
| Weight, kg | 65.1 [50-111] |
| NYHA class | |
| I | 8 (34.8) |
| II | 10 (43.4) |
| III | 5 (21.7) |
| Peak VO2, mL/mn/kg | 21.7±6.5 |
| BNP, pg/dL | 86.8±85.3 |
| Length of QRS, ms | 140±28 |
| Congenital heart disease | |
| Tetralogy of Fallot | 20 (87.0) |
| Pulmonary valve stenosis | 2 (8.7) |
| Ross procedure | 1 (4.3) |
| Age at main surgery, mo | 31 [1-348] |
| RVEDV, mL/m2 | 138±28 |
| RVESV, mL/m2 | 81±15 |
| RVEF, % | 43.5±7.5 |
| Pulmonary regurgitant fraction (2D PC), % | 51.9±10.1 |
NYHA, New York Heart Association; PC, phase contrast; RVEDV, right ventricular end diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end-systolic volume.
Data are expressed as no. (%), mean ± standard deviation or median [interquartile range].
Primary successful PPVI was performed in 18 of 23 patients. Three patients underwent PPVI with an Edwards XT 29 mm valve, 8 patients underwent classic Melody 22 mm valve implantation, and the others underwent various techniques for Melody valve implantation in a large RVOT (jailing or unconventional method). In 1 patient, the RVOT was too large for a Melody and PPVI was performed in a second stage with a SAPIEN XT 29 mm.
Accuracy of 4D flow diameter measurements on phantomCorrelations and Bland-Altman plots are provided in figure 3. Sizing plate ranged from 4 to 38 mm diameters. The mean difference±SD between the 4D flow and phantom diameter estimates was 0.2±0.2 mm, with a maximal overestimation of up to 0.6 mm for a diameter ranging between 30 and 40 mm (1.7%). Overall 4D flow measurements were accurate and highly reliable with sizing plate in vitro.
Accuracy of 4D flow measure using a sizing plate for atrial septal defect. Upper layer: sizing plate from 4D flow MRI. Lower layer: linear regression analysis and Bland-Altman analysis for comparison between sizing plate and 4D flow MRI. MRI, magnetic resonance imaging; SD, standard deviation.
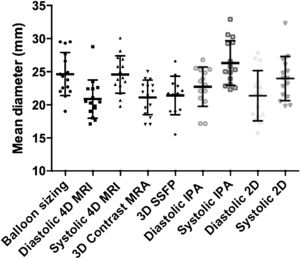
Plots of the RVOT diameter estimates between MRI sequences, as well as between systole and diastole, are provided in figure 4. The mean RVOT diameter was measured as 25.4±4.3 mm by balloon sizing, 25.6±3.8 mm, and 21.8.3±3.6 mm by 4D flow MRI in systole and diastole, respectively.
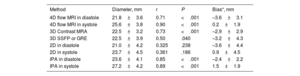
Comparison between magnetic resonance imaging and catheterization-derived measurementsCorrelations between each modality of measurement and balloon sizing are presented in table 2. 2D in diastole and systole were not significantly correlated to balloon sizing (P=.24 and P=.19, respectively).
Comparison of 4D flow, 3D angio-MRI, 3D SSFP, 2D and angiography compared with balloon sizing as the reference method
| Method | Diameter, mm | r | P | Bias*, mm |
|---|---|---|---|---|
| 4D flow MRI in diastole | 21.8±3.6 | 0.71 | <.001 | –3.6±3.1 |
| 4D flow MRI in systole | 25.6±3.8 | 0.90 | <.001 | 0.2±1.9 |
| 3D Contrast MRA | 22.5±3.2 | 0.73 | <.001 | –2.9±2.9 |
| 3D SSFP or GRE | 22.5±3.9 | 0.50 | .040 | –3.2±4.3 |
| 2D in diastole | 21.0±4.2 | 0.325 | .238 | –3.6±4.4 |
| 2D in systole | 23.7±4.5 | 0.361 | .186 | 0.9±4.5 |
| IPA in diastole | 23.6±4.1 | 0.85 | <.001 | –2.4±2.2 |
| IPA in systole | 27.2±4.2 | 0.89 | <.001 | 1.5±1.9 |
GRE, gradient echo; IPA, invasive pulmonary angiography; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; SD, standard deviation; SSFP: steady-state free precession.
Unless otherwise stated, the results are expressed as mean±standard deviation.
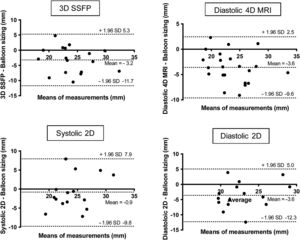
Linear regression and Bland-Altman plots are provided for 4D flow MRI in systole, 3D contrast MRA and invasive angiography in systole and diastole, compared with balloon sizing (figure 5).
Linear regression analysis and Bland-Altman analysis for comparison between systolic 4D MRI, 4D MRA, systolic and diastolic angiography compared with balloon sizing; IPA, invasive pulmonary angiography; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; SD, standard deviation.
Bland-Altman analysis (figure 5) confirmed the lack of significant bias, with relatively narrow 95% limits of agreement for systolic 4D flow. The magnitude of differences observed was moderate, as reflected in 95% of values ranging between –3.5 and 3.9 mm. Although the correlation between systolic and diastolic angiography, 3D contrast MRA, diastolic 4D MRI, and 3D SSFP were good or moderate, the Bland-Altman analysis showed a higher bias (+1.5 mm, 2.4 mm, –2.9 mm, 3.6, and 3.2 mm respectively) with wide 95% limits of agreement (–2.3 to 5.3 mm; –6.7 to 2.0 mm; –8.7 to 2.8 mm; –11.7 to 5.3 mm, and –9.6 to 2.5 mm, respectively) (figure 5 and figure 6).
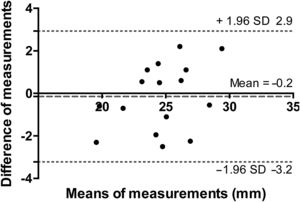
Systolic 4D flow interobserver reproducibilityInterobserver reproducibility of 4D flow measurement estimates was very good with an ICC of 0.92 (95% confidence interval [95%CI], 0.77-0.97; P <.0001). Bland-Altman analysis confirmed the lack of significant bias since it was –0.2±1.5 mm (figure 7).
DISCUSSIONIn this study, 4D flow MRI sequence appeared to be an accurate tool to measure the native RVOT before PPVI, overperforming measurements from 3D contrast MRA and 3D SSFP/GRE, with a systolic measurement close to balloon sizing. In addition, as the RVOT size changes throughout the cardiac cycle, the timing of the measurement is crucial, and we found that systolic measurement both in 4D flow or invasive angiography were the closest to balloon sizing. As the 4D flow is increasingly used in MRI examination16 when evaluating patients with repaired tetralogy of Fallot to assess pulmonary regurgitation and possible associated functional stenosis along the RVOT, our results encourage the additional use of anatomical information to better estimate the maximal systolic size of this RVOT before planning PPVI (figure 8).
Central illustration. 4D flow MRI sequence appears as an accurate tool to measure the native RVOT before PPVI, outperforming measurements from other MRI modalities, with a systolic measurement close to balloon sizing. GRE, gradient echo; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; PPVI, percutaneous pulmonary valve implantation; RVOT, right ventricular outflow tract; SSFP, steady-state free precession.
As well as MRI, other imaging modalities are systematically performed by some centers for sizing RVOT. CT has excellent spatial resolution and allows an accurate estimate of RVOT size and anatomy. However, to assess the heart throughout the entire cardiac cycle or even using a prospectively gated scan, the radiation exposure often requires more than 1 mSv of x-ray radiation.17 Such questions about the risk of radiation are crucial in patients with CHD. Cohen et al.18 found that increasing exposure to low-dose ionizing radiation from cardiac imaging in adults with CHD raised concerns about the life-long risk of malignancy. Interestingly, a large number of procedures (≥ 6 procedures, including CT) were associated with a substantially elevated risk of cancer (OR, 3.08; 95%CI, 1.77-5.37). Nevertheless, the numbers of low-dose ionizing radiation exposures and exposures occurring at younger ages from 1990 to 2005 in patients with CHD is increasing.19 To date, guidelines encourage avoiding CT scanning as much as possible.4
Our study provides dynamic information, with 4D flow MRI, about the changes of RVOT during the cardiac cycle. An increase of up to 20% of RVOT was found here in systole, as previously shown in a 4D CT study.20 These findings are important because most MRA and whole-heart cardiac MRI acquisitions are often acquired predominantly in diastole or even without ECG gating to reduce acquisition time and the duration of breath-hold.21 Recently, a few studies have confirmed the need to perform the acquisition in systole.21,22 2D SSFP showed very poor performance, which was expected since acquisition planes were located during before MRI scanning in the setting of complex RVOT shapes moving in and out of the planes through the cardiac cycle. Angio MRA and, to an even greater extent, 3D SSFP/GRE, also showed disappointing results in our study, which in the case of angio MRA can be attributed to the absence of ECG gating and measurements in the diastolic period for 3D SSFP.22 The use of a systolic ECG-gated SSFP volume would likely lead to better results but was not evaluated in our study.22
Other MRI techniques could be an alternative to 4D flow to provide a volumetric time-resolved acquisition of the RVOT. Time-resolved SSFP sequence will require further validation and is not widely available.23 However, performing either a dedicated systolic or time-resolved 3D SSFP acquisition of the RVOT would cost extra acquisition time in already long MRI protocols in the setting of CHD. On the other hand, 4D flow tends to be already included in MRI protocols in patients with repaired Tetralogy of Fallot and provide useful information related to flow.24–26 By using post contrast 4D flow magnitude images to estimate RVOT geometry, other dedicated acquisitions for RVOT sizing are not necessary, thus simplifying workflow. The higher accuracy of 4D flow systole measurements was not surprising because during the RV ejection during systole, the quality of the phase, and module images are much better than during diastole when velocities are much lower, thus leading to higher noise and lower image quality.
Lastly, another proposed solution is a 3D cardiac printed model to better understand anatomy and to predict PPVI.27,28 However, the spatial resolution of 4D flow MRI is lower than CT, which limits its use for 3D cardiac printing.29 In addition, the fusion of different imaging techniques shows potential to guide the interventions, but CT remains more suitable than MRI in this indication at the cost of radiation exposure.30
Accurate sizing of RVOT to plan PPVI will be even more important in the future considering that one of the major challenges is to expand PPVI to a broader population of patients, especially in those with large native RVOT. Thus, the Venus P valve, a self-expanding valve (Venus Medtech, China) recently received CE marking,31 while the Medtronic Harmony TPV, a self-expanding valve (Medtronic, United States) received Food and Drug Administration approval in 2021.32 Other devices are under assessment such as the anchoring adaptor for the 29 mm SAPIEN 3 Alterra Adaptive Prestent (Edwards Lifesciences, United States).33 These new devices could theoretically allow PPVI to be used for native RVOT up to 40 mm and would still require an accurate assessment of the size of the native RVOT before PPVI. Nevertheless, even if the reliability of the measure with 4D flow MRI was appropriate in the current range of system values, it needs to be confirmed for larger values.
LimitationsFirst, despite homogeneous clinical characteristics, our study population was from a single center and the sample size was limited size. Even though accuracy was good and we found a good correlation with the balloon sizing, the reproducibility of the measures may raise some concerns. By design, this study did not assess the predictive value of measurements on PPVI success.
Because balloon sizing was the reference standard, only patients referred to catheterization were included in this study. A potential bias could be observed since only patients found suitable for PPVI based on the standard of care were included, after we excluded those whose RVOT was deemed too large. We were also unable to analyze the performance of the 4D flow to exclude patients with a very large RVOT, which is still a contraindication for interventional procedures.34
Another concern is the period of analysis since we changed the protocol to refer patients to catheterization from 2019, using 4D MRI. Therefore, we included patients before this time, allowing a comparison between 4D MRI and balloon sizing and limiting the bias.
CONCLUSIONSAnatomic information from 4D flow MRI is accurate to measure RVOT before PPVI and outperforms standard MRI sequences. The size of RVOT changes throughout the cardiac cycle and we found that systolic measurements were closer to the balloon sizing when used as the reference. Further studies are needed to evaluate 4D flow measurements to predict PPVI outcomes and the success of the implementation.
FUNDINGThe authors state that this work has not received any funding.
AUTHORS’ CONTRIBUTIONSC. Karsenty conceived the work, performed the acquisition and the analysis, wrote the manuscript and approved the final version. E. Mousseaux conceived the work, revised the intellectual content and approved the final version of the manuscript. Y. Alattar performed the acquisition and the analysis, and approved the final version. G. Marcilhacy performed the acquisition and the analysis, and approved the final version of the manuscript. U. Gencer performed the acquisition and the analysis, and approved the final version. L. Iserin conceived the work, revised the intellectual content and approved the final version of the manuscript. M. Ladouceur conceived the work, revised the intellectual content and approved the final version of the manuscript. A. Legendre performed some acquisition, revised the intellectual content and approved the final version of the manuscript. M. Laredo performed some acquisition and analysis, revised the intellectual content and approved the final version of the manuscript. D. Bonnet performed some acquisition revised the intellectual content and approved the final version of the manuscript. S. Malekzadeh-Milani performed some acquisition revised the intellectual content and approved the final version of the manuscript. G. Soulat conceived the work, performed the acquisition and the analysis, wrote the manuscript and approved the final version.
CONFLICTS OF INTERESTThe authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
- -
Magnetic resonance imaging (MRI) including 4D flow is used to assess the right ventricle and pulmonary arteries.
- -
MRI is useful before percutaneous pulmonary valve implantation for pulmonary regurgitation.
- -
Various MRI sequences including 4D flow are used but the added value remains unknown.
- -
4D flow MRI sequence is accurate to measure RVOT before percutaneous pulmonary valve implantation.
- -
4F flow MRI outperformed standard MRI sequences to measure RVOT.
- -
In patients with RVOT dysfunction, the RVOT must be measured in systole.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.02.010