Pulmonary regurgitation is a common complication in patients with repaired tetralogy of Fallot or congenital pulmonary stenosis. Electrocardiographic variables have been correlated with parameters used to evaluate right ventricular function. We aimed to analyze the diagnostic value of the width and fragmentation of the electrocardiogram in the identification of patients with right ventricular dysfunction and/or dilation.
MethodsWe selected 107 consecutive patients diagnosed with severe pulmonary insufficiency after repair of pulmonary stenosis or tetralogy of Fallot. The tests included electrocardiography, echocardiography, and magnetic resonance. Each electrocardiogram was analyzed manually to measure QRS duration. We defined QRS fragmentation as the presence of low-voltage waves in the terminal portion of the QRS complex in at least 2 contiguous leads.
ResultsWe found a significant negative correlation between QRS width and right ventricular function, as well as a positive correlation with right ventricular volume. The receiver operating characteristic curve indicated a cut-off point for QRS width of 140ms, which showed good sensitivity for a diagnosis of right ventricular dilation (> 80%) and dysfunction (> 95%). In logistic regression models, a QRS duration > 140ms was found to be the only independent predictor of right ventricular dilation and dysfunction.
ConclusionsElectrocardiography is a rapid, widely available, and reproducible tool. QRS width constitutes an independent predictor of the presence of right ventricular dilation and dysfunction. This study is the first to provide a cutoff value for QRS width to screen for right ventricle involvement.
Keywords
Pulmonary regurgitation (PR) is a common complication in patients who underwent surgery for tetralogy of Fallot (TOF) or for congenital pulmonary stenosis as children. It has been shown that surgical correction of TOF ameliorates the symptoms and prolongs survival.1,2 In pulmonary stenosis, the obstruction can be valvular, subvalvular (infundibular), or supravalvular. Valvular stenosis can be treated by means of percutaneous valvuloplasty, whereas supravalvular and subvalvular stenosis generally require conventional surgery, and the prognosis is less benign.3
The severe PR that usually develops after the intervention leads to dilation and progressive dysfunction of the right ventricle (RV), increasing the risk of arrhythmias and worsening the prognosis of these patients. Treatment is surgical, and involves replacement of the pulmonary valve with a prosthesis or homograft. When pulmonary valve replacement is carried out at the proper time, it is usually accompanied by a reduction in RV volume and, on occasion, by an improvement in the right ventricular ejection fraction (RVEF). However, when the intervention is indicated late after TOF repair, RV recovery is incomplete.4 The current indications for pulmonary valve replacement are not clearly defined, and the criteria are mainly based on the development of clinical events and/or excessive RV dilation.5 Thus, the proper timing of surgery is a challenge for clinicians as, in addition to the clinical variables, data from imaging studies must also be considered.
Cardiac magnetic resonance (CMR) is the gold standard for estimating RVEF and RV volumes. However, CMR is a very costly technique that is not available in all centers. Moreover, its use is controversial in patients with cardiac pacing devices or defibrillators, especially the latter, because of the risk of interferences and signal loss they induce.6 In this context, noninvasive complementary diagnostic methods, such as electrocardiography (ECG), become especially important as possible indirect markers of the progression of heart disease.
Surface ECG is a simple, affordable, and virtually noninvasive diagnostic test; different electrocardiographic variables have been correlated with parameters of RV function and with the onset of cardiovascular events.7,8 The relationship between QRS fragmentation and width and clinical parameters, including the development of arrhythmias, has been studied in TOF.9
The objective of this study was to analyze the diagnostic value of ECG parameters to identify those patients with RV dysfunction and/or dilation secondary to severe PR.
METHODSStudy PopulationWe carried out a retrospective selection of 107 patients diagnosed as having severe PR who were being followed up in the adult congenital heart disease unit of our center. All the patients had been diagnosed with TOF or pulmonary stenosis and had undergone surgical repair as children, resulting in significant PR as a sequel. We defined severe PR in accordance with the criteria previously established in the literature.10
The variables recorded included demographic and clinical data. Surface ECG, transthoracic echocardiography, and CMR were performed as part of the routine clinical evaluation. The interval between ECG and CMR was not longer than 15 days in any of the patients. We excluded patients with 1 or more of the following conditions: a) use of medication affecting QRS duration; b) pacemaker to stimulate cardiac rhythm; c) problems with the interpretation of CMR data due to the region of signal loss owing to the presence of an implanted cardioverter defibrillator; and d) presence of tricuspid atresia and/or RV hypoplasia.
Electrocardiographic EvaluationA 12-lead resting ECG was performed in all the patients as part of their usual follow-up using a digital acquisition and storage system (filter band, 0.16Hz to 100Hz; 25mm/s; 10mm/mV; PageWriter TC70 cardiograph, Philips Medical Systems; Eindhoven, The Netherlands). To obtain the measurements, the size was augmented and a specific software package was employed (Cardio Calipers® 3.3, Iconico) with a resolution of 1ms on the horizontal axis and 0.01mV on the vertical axis.
Manual analysis of each ECG included measurement of the duration of the QRS complex in each of the precordial leads and calculation of the arithmetic mean. QRS duration was defined as the distance between the first deflection and the point of confluence of the final vector with the isoelectric line. We excluded measurement of atrial and ventricular premature beats. For the statistical analysis, we defined long QRS as that with a duration > 120ms.
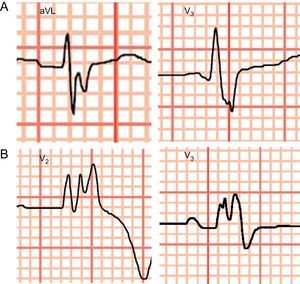
We analyzed QRS fragmentation (QRSf), defined as the presence of notches or low-voltage waves (R’) in the terminal portion of the QRS complex or at the beginning of the ST segment in at least 2 contiguous leads (Figure 1A). In patients with prolonged QRS (> 120ms), even with bundle branch block morphology, QRS fragmentation was defined as the presence of more than 2 R’ in the R wave or in the nadir of the S wave in at least 2 contiguous leads (Figure 1B).
All of the ECG were analyzed by clinical cardiologists who were blind to the results of CMR imaging. Ten patients were randomly selected to repeat the analysis of the QRS complex, this time blinded, by the same operator and by a second operator to establish the interobserver and intraobserver variability.
Cardiac Magnetic ResonanceAll the CMR images were acquired using a 1.5-T Magnetom scanner with Syngo MR 2004V® software (Siemens Medical Solutions; Elangen, Germany) and were interpreted by a cardiologist who is an expert in cardiac imaging. With the protocol used, we obtained cine sequences using true fast imaging with steady state precession (True FISP), morphological sequences (T1 and T2-weighted turbo spin echo [TSE] images), and viability sequences with turbo fast low angle shot (FLASH) images. The data on the volumes and ejection fractions of both ventricles were analyzed using the QMASS® MR 6.1.5. software package (Medis; Leiden, The Netherlands). We used CMR as the gold standard for the calculation of the RVEF (using Simpson's method) and systolic and diastolic ventricular volumes. In accordance with current recommendations, we defined RV dysfunction as the presence of a RVEF<45%.11 We defined RV dysfunction as the presence of a RV end-diastolic volume index > 150mL/m2.
Statistical AnalysisWe obtained descriptive statistics for the frequency of the continuous variables studied (mean [standard deviation]) and the categorical values (percentage [standard deviation]). To determine interobserver and intraobserver variability, we used the intraclass correlation coefficient for continuous variables and the kappa coefficient for categorical variables. Simple linear correlation studies were carried out with determination of the Pearson correlation coefficient between continuous variables. Comparisons of the means were analyzed using Student's t test. On the basis of the receiver operating characteristic (ROC) curve, we estimated the optimal cutoff points for each parameter for the diagnosis of RV dysfunction and dilation, with their corresponding sensitivity, specificity, and positive and negative predictive values. Logistic regression models were employed to determine the factors predictive of RV dilation or dysfunction.
The statistical analysis was performed with the IBM SPSS® statistical software package (SPSS Inc.; Chicago, United States). P values < .05 were considered to indicate statistical significance.
RESULTSStudy PopulationOf the 114 patients with severe PR followed up in our adult congenital heart disease unit, we included 107 in the analysis. Of these 107 patients, 7 were excluded for the following reasons: 3 because they had another associated congenital anomaly; 1 due to paced ventricular rhythm; and 3 because they had an implantable cardioverter defibrillator, since the artifact caused by this device impedes interpretation of CMR images.
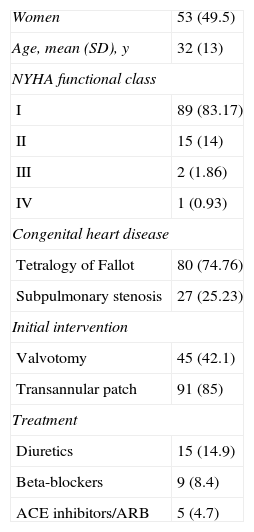
The characteristics of the study population are shown in Table 1. In all, 49.5% of the patients were women, with a mean age of 32 years. Baseline disease consisted of TOF in 80 patients (74.7%) and subpulmonary stenosis in 27 (25.23%). The surgical technique used was RV outflow tract enlargement through a transannular patch in 91 patients (85%) and pulmonary valvotomy in 45 (42.1%). In the overall group of patients, 89 (83.17%) were in New York Heart Association (NYHA) class I, 15 (14%) were in class II, 2 (1.86%) were in class III, and 1 (0.83%) was in class IV. Drug therapy consisted of an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker in 5 patients (4.7%), beta-blockers in 9 (8.4%), and loop diuretic (furosemide) in 15 (14.9%).
Patient Characteristics (n=107)
| Women | 53 (49.5) |
| Age, mean (SD), y | 32 (13) |
| NYHA functional class | |
| I | 89 (83.17) |
| II | 15 (14) |
| III | 2 (1.86) |
| IV | 1 (0.93) |
| Congenital heart disease | |
| Tetralogy of Fallot | 80 (74.76) |
| Subpulmonary stenosis | 27 (25.23) |
| Initial intervention | |
| Valvotomy | 45 (42.1) |
| Transannular patch | 91 (85) |
| Treatment | |
| Diuretics | 15 (14.9) |
| Beta-blockers | 9 (8.4) |
| ACE inhibitors/ARB | 5 (4.7) |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; NYHA, New York Heart Association; SD, standard deviation.
Data are expressed as No. (%) or mean (standard deviation).
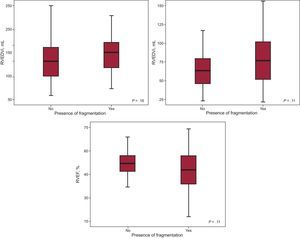
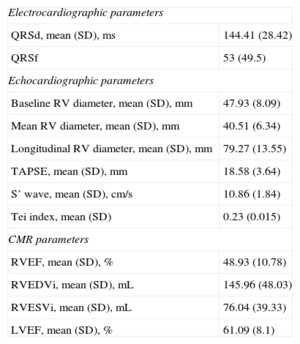
All of the patients underwent echocardiography within 15 days after CMR. The echocardiographic data and those obtained from CMR, the gold standard used in our study, appear in Table 2. A total of 33 patients (30.8%) had RV dysfunction according to CMR criteria (RVEF<45%). The mean (standard deviation) tricuspid annular plane systolic excursion (TAPSE) was 18.58 (3.64) mm; the S’ wave, 10.86 (1.84) cm/s; and the Tei index, 0.23 (0.015). The RVEF estimated by CMR was 48.93% (10.78%). CMR calculation of RV volumes showed that 49 patients (45.8%) had an end-systolic volume index > 150mL (taken as the cutoff point for significant RV dilation).
Echocardiographic and Cardiac Magnetic Resonance Data
| Electrocardiographic parameters | |
| QRSd, mean (SD), ms | 144.41 (28.42) |
| QRSf | 53 (49.5) |
| Echocardiographic parameters | |
| Baseline RV diameter, mean (SD), mm | 47.93 (8.09) |
| Mean RV diameter, mean (SD), mm | 40.51 (6.34) |
| Longitudinal RV diameter, mean (SD), mm | 79.27 (13.55) |
| TAPSE, mean (SD), mm | 18.58 (3.64) |
| S’ wave, mean (SD), cm/s | 10.86 (1.84) |
| Tei index, mean (SD) | 0.23 (0.015) |
| CMR parameters | |
| RVEF, mean (SD), % | 48.93 (10.78) |
| RVEDVi, mean (SD), mL | 145.96 (48.03) |
| RVESVi, mean (SD), mL | 76.04 (39.33) |
| LVEF, mean (SD), % | 61.09 (8.1) |
CMR, cardiac magnetic resonance; LVEF, left ventricular ejection fraction; QRSd, QRS duration; QRSf, QRS fragmentation; RV, right ventricular; RVEDVi, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end-systolic volume index; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
Data are expressed as No. (%) or mean (standard deviation).
First, we carried out a study of the reproducibility of the electrocardiographic data by analyzing interobserver and intraobserver variability. The interclass correlation coefficient for intraobserver correlation was 0.984 for the estimation of the QRS duration and was 0.986 for the calculation of the QRS duration when interobserver correlation was examined. These findings correspond to excellent interobserver and intraobserver correlations. There was excellent reproducibility in the detection of QRS fragmentation (κ=1) for both interobserver and intraobserver agreement.
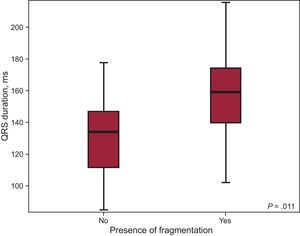
The duration of the QRS complex patients included in the sample ranged from 84.67ms and 215.67ms, with a mean (standard deviation) of 144.41 (28.42) ms; 79 patients (73.8%) had a prolonged QRS duration (> 120ms). With respect to intraventricular conduction, 79 patients (73.8%) had complete right bundle branch block. QRS fragmentation was detected in 53 patients (49.5%). Most of the patients with fragmentation also had a prolonged QRS (48 patients; 90.56%), and the difference between the QRS duration in patients showing fragmentation and those who did not was statistically significant (QRS duration, 156.83ms vs 131.5ms; P<.001) (Figure 2).
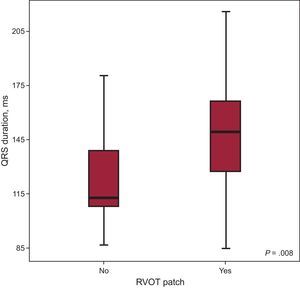
When the electrocardiographic findings were analyzed in relation to the surgical technique employed, we observed that, in those patients whose intervention had involved the use of a transannular patch, the width of the QRS complex was significantly greater (QRS duration, 147.67ms vs 126.48ms; P=.008) (Figure 3). However, with regard to the presence of fragmentation, there were no significant differences between patients with and without a transannular patch.
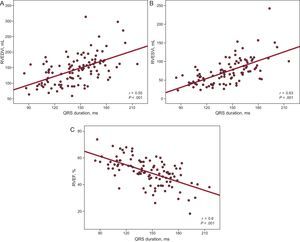
Study of the Correlation Between Electrocardiographic Parameters and Imaging DataWe first studied whether or not there was a correlation between QRS width and the parameters for RV volume and function estimated by CMR (Figures 4A-C). The Pearson correlation demonstrated a significant negative correlation between QRS duration and RVEF (r=–0.6; P<.001) and a significant positive correlation between QRS duration and the RV end-systolic volume index (r=0.63; P<.001) and between QRS duration and the RV end-diastolic volume index (r=0.55; P<.001), findings that indicate that the greater the QRS duration, the lower the RVEF and the greater the RV volumes.
Study of the correlation between QRS width and volume parameters and right ventricular function estimated by means of cardiac magnetic resonance. RVEDVi, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end-systolic volume index.
The comparison between the groups of patients with and without fragmentation revealed no statistically significant differences in RV end-diastolic volume index (137.68mL vs 150.49mL; P=.16), RV end-systolic volume index (67.79mL vs 79.36mL; P=.11), or RVEF (51.12% vs 47.88%; P=.11). Nevertheless, we observed a trend toward greater RV dilation and poorer systolic function among patients whose ECG revealed fragmentation (Figure 5).
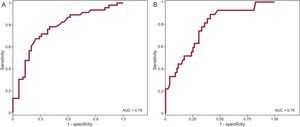
A receiver operating characteristic curve (Figure 6A) was created to enable us to determine the discriminatory power of the parameter, QRS duration, for the detection of RV dysfunction. The area under the curve obtained for QRS duration was 0.79. The cutoff point of QRS duration > 140ms showed a sensitivity of 89% (95% confidence interval [95%CI], 72%-96%) and a specificity of 56% (95%CI, 45%-66%) for the prediction of RV dysfunction, with a negative predictive value of 94% (95%CI, 81%-98%). We studied the receiver operating characteristic curve obtained using QRS duration for the detection of RV dilation (> 150mL/m2) and found that a QRS > 140ms had a sensitivity of 76% (95%CI, 63%-85%) and a specificity of 65% (95%CI, 52%-77%), with a negative predictive value of 72% (95%CI, 58%-83%). The area under the curve for the detection of RV dilation was 0.76 (Figure 6B).
With the data derived from the receiver operating characteristic curve, we established a QRS width > 140ms as the optimal cutoff point for the detection of RV dilation and dysfunction, thus creating a binary variable based on whether the QRS duration was longer or shorter than 140ms. We employed this new variable and the previously recorded data (age, sex, age at surgical correction, time elapsed since the correction, previous palliative shunt placement, use of a transannular patch in the surgical procedure, and presence of fragmentation) to design logistic regression models for the prediction of the presence of RV dilation and dysfunction. The only independent predictors of RV dilation were QRS duration > 140ms (hazard ratio [HR]=6.69; 95%CI, 2.69-16.71; P<.001) and male sex (HR=0.34; 95%CI, 0.14-0.84; P=.019). Both variables also proved to be independent predictors of RV dysfunction: QRS duration > 140ms, HR=10.69 (95%CI, 2.91-39,37; P<.001); male sex, HR=0.32 (95%CI, 0.12-0.895; P=.031).
DISCUSSIONSurface ECG is an essential technique in clinical cardiology; it is a noninvasive, inexpensive tool that is available in nearly every health care center. It is of immense clinical value in the diagnosis of a wide range of cardiac disorders12 and also provides prognostic information.13
In our report, we propose consideration of the usefulness of QRS width and fragmentation in the diagnosis of RV dilation and dysfunction. We carried out a selective study of patients with significant PR after surgical repair of TOF or congenital pulmonary stenosis.
Previous studies have demonstrated the value of QRS complex duration and the presence of fragmentation for the prediction of events, as well as their good correlation with clinical and imaging parameters in patients with heart disease, whether acquired14,15 or congenital.16,17 The relationship between QRS complex duration and RV volume has been studied previously; Gatzoulis et al18 demonstrated that the presence of a QRS > 180ms correlates with more marked RV dilation and is able to predict the development of ventricular arrhythmias in patients with repaired TOF. According to Gatzoulis et al, the abnormal depolarization produced with dilated RV leads to QRS widening, a hypothesis they base on a mechanoelectrical interaction; later studies have confirmed this hypothesis by examining RV dynamics using 3-dimensional echocardiography19 and CMR.20 In a recent review, Bassareo and Mercuro9 report that QRS duration is one of the most powerful predictors of ventricular arrhythmias in patients with repaired TOF, and they focus on its measurement. As a limitation, they point out the marked interobserver and intraobserver variabilities in QRS measurement.
Sung et al21 proposed that certain electrocardiographic patterns and prolonged QRS in patients with ostium secundum atrial septal defect were related to RV dilation, rather than to an intrinsic disturbance of the intraventricular conduction system. Recently, Ladouceur et al22 studied the role of the QRS complex in patients with significant PR associated with congenital heart disease. The authors of that study concluded that certain echocardiographic and electrocardiographic parameters (QRS width) facilitate the identification of patients requiring closer follow-up by means of CMR.
QRS fragmentation has been established as a marker of myocardial involvement, which can take the form of a primary cardiac disorder (such as left ventricular hypertrophy,12 an acute coronary syndrome,14 or arrhythmogenic RV dysplasia15), or a secondary disorder, in the context of systemic disease (such as amyloidosis,15 rheumatoid arthritis,16 or pulmonary sarcoidosis17).
In the setting of congenital heart disease in adults, there are a number of recent publications on fragmentation; in a recent study, Assenza et al17 reported that the presence of fragmentation predicted a greater “atrialized” RV volume in a cohort of patients with Ebstein anomaly. Shanmugam et al23 studied fragmentation in patients with repaired TOF; it was associated with a dysfunctional RV and the existence of RV outflow tract aneurysm (particularly fragmentation in anterior leads).
Our study was performed in patients diagnosed with significant PR secondary to surgical repair of TOF or with congenital pulmonary stenosis. In contrast to the report by Bassereo and Mercuro,9 study of the parameters derived from the QRS complex showed a high degree of reproducibility in both the interobserver and intraobserver electrocardiographic measurements. Our findings are similar to those previously reported in the literature in that the QRS width showed a good correlation with the data on RV function and volume estimated by CMR, and was an independent predictor of the presence of RV dilation and dysfunction. Likewise, the area under the receiver operating characteristic curves showed the accuracy of QRS width for the detection of these 2 conditions to be good.
As a novel element of this study, on the basis of the data obtained from the receiver operating characteristic curves, we selected an optimal cutoff point to screen for the presence of RV dilation and dysfunction. In fact, a QRS width > 140ms showed a sensitivity > 90% for the detection of RV dysfunction and > 80% for the detection of RV dilation.
The incidence of QRS fragmentation in our sample was lower than that reported in previous studies (approximately 50% of the patients, vs nearly 80% in other published series); moreover, in contrast to observations reported in the literature,23 it was not found to have a statistically significant relationship to RV dilation and dysfunction. A possible explanation for this finding lies in the differences between the study populations. For example, the patients included in the report by Shanmugam et al,23 who recorded a higher prevalence of fragmentation, had more marked RV dilation and poorer systolic function. The role of QRS fragmentation in patients with a reduced RV volume is probably yet to be defined, although, in view of our results, it may contribute little in the initial phases of ventricular dilation and dysfunction. It is precisely in these early phases when screening for RV dysfunction is especially important.
In our regression analysis, we found that the presence of a QRS > 140ms was an independent predictor of RV involvement; the fact that fragmentation was not is probably related to the marked collinearity between the 2 variables.
LimitationsThe most important limitation of this study is the small sample size (107 patients), which restricts extrapolation of the conclusions obtained, although we are aware of the difficulty involved in enrolling a large number of patients in studies on diseases of low prevalence. In addition, this report is based on retrospective data, which increases the risk of producing biases.
We consider that additional larger scale studies are necessary to confirm the value of QRS width in screening for RV dilation and/or dysfunction.
We suggest the need to study the diagnostic value of fragmentation in patients with a moderately increased RV volume, as the data provided in the literature comes from studies carried out in patients with marked RV dilation.
CONCLUSIONSThis is the first study to propose a cutoff point for QRS duration for the screening of RV involvement. This parameter is able to independently predict the presence of RV dilation and dysfunction. The low variability of the electrocardiographic measurements make it a useful, rapid, available, and reproducible tool.
CONFLICTS OF INTERESTNone declared.