Sodium-glucose cotransporter-2 inhibitors (SGLT2i) induce short-term changes in renal function and hemoglobin. Their pathophysiology is incompletely understood. We aimed to evaluate the relationship between 1- and 3-month estimated glomerular filtration rate (eGFR) and hemoglobin changes following initiation of dapagliflozin in patients with stable heart failure with reduced ejection fraction (HFrEF).
MethodsThis is a post hoc analysis of a randomized clinical trial that evaluated the effect of dapagliflozin on 1- and 3-month peak oxygen consumption in outpatients with stable HFrEF (DAPA-VO2 trial, NCT04197635). We used linear mixed regression analysis to assess the relationship between eGFR and hemoglobin changes across treatment arms.
ResultsA total of 87 patients were evaluated in this substudy. The mean age was 67.0± 10.5 years, and 21 (24.1%) were women. The mean baseline eGFR and hemoglobin were 66.9±20.7mL/min/1.73m2 and 14.3±1.7g/dL, respectively. Compared with placebo, eGFR did not significantly change at either time points in the dapagliflozin group, but hemoglobin significantly increased at 1 and 3 months. At 1 month, the hemoglobin increase was related to decreases in eGFR only in the dapagliflozin arm (P <.001). At 3 months, there was no significant association in either treatment arms (P=.123). Changes in eGFR were not associated with changes in peak oxygen consumption, quality of life, or natriuretic peptides.
ConclusionsIn patients with stable HFrEF, 1-month changes in eGFR induced by dapagliflozin are inversely related to changes in hemoglobin. This association was no longer significant at 3 months.
Keywords
Prior evidence shows that sodium-glucose cotransporter-2 inhibitors (SGLT2i) led to a short-term estimated glomerular filtration rate (eGFR) reduction in some patients.1 Although most of these changes were mild, transient, and not associated with worse outcomes,1,2 these changes generated uncertainty and, sometimes, a reason for treatment withdrawal.3
Short-term hemoglobin and hematocrit increase is another common finding following SGLT2i initiation,4 and has been associated with favorable clinical responses mediating the benefit attributable to SGLT2i in type 2 diabetes and heart failure (HF).5 A short-term increase in hemoglobin is a well-recognized proxy of hemoconcentration in patients with HF treated with diuretics.6 Due to the aquaretic properties of SGLT2i, hemoglobin increase following SGLT2i initiation has also been postulated as a marker of hemoconcentration, especially in the short-term.7,8 Likewise, in patients with HF treated with a vigorous diuretic strategy, there is cumulative evidence endorsing the role of creatinine increases/eGFR decreases as markers of hemoconcentration rather than true renal function impairment, especially when changes are mild and transient.1,2 Thus, we postulated that a decrease in renal function following SGLT2i may be related to hemoglobin increase as a marker of hemoconcentration.
In this study, we aimed to evaluate the association between changes in eGFR (ΔeGFR) and hemoglobin (ΔHb) at 1 and 3 months following dapagliflozin initiation in patients with stable HF with reduced ejection fraction (HFrEF).
METHODSStudy sampleThis is a post hoc analysis of the DAPA-VO2 randomized clinical trial,9 an investigator-initiated, multicenter, double-blind, randomized clinical trial designed to evaluate the effect of dapagliflozin on maximal functional capacity assessed by peak oxygen consumption (peakVO2) in outpatients with stable HFrEF. The patients were randomized 1:1 to receive either dapagliflozin or a placebo. PeakVO2 was evaluated at baseline, and at 1 and 3 months. This study was approved by the Agencia Española del Medicamento y Productos Sanitarios (AEMPS) and by the Comité Ético de Investigación Clínica (CEIC) of our center. This study was registered at ClinicalTrials.gov (NCT04197635). All patients signed an informed consent form. The study population included patients with stable chronic HF (New York Heart Association [NYHA II–III/IV]) and left ventricular ejection fraction ≤ 40%. The eligibility criteria, study procedures, and main findings have been published elsewhere.9 Patients with baseline or follow-up conditions affecting hemoglobin levels were excluded from this analysis (2 patients with gastrointestinal bleeding and 1 receiving methotrexate).
ProceduresRandomized patients performed a baseline, 1 and 3-month cardiopulmonary exercise test using incremental and symptom-limited cardiopulmonary exercise testing on a bicycle ergometer. Maximal functional capacity was defined as the point when the patient stopped pedaling because of symptoms, and the respiratory exchange ratio (RER) was ≥ 1.05. PeakVO2 was defined as the highest value of VO2 during the last 20seconds of exercise. During the same study visits, a 6-minute walk test, echocardiography, and quality of life assessment (Minnesota Living with Heart Failure Questionnaire, [MLHFQ]) were performed, and blood samples were obtained. The eGFR was calculated by using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.10 N-terminal pro-B-type natriuretic peptide (NT-proBNP) was analyzed with standard commercial enzyme immune analysis (Elecsys NT-proBNP assay, Roche Diagnostics, Switzerland). None of the patients received intravenous iron or erythropoietin-stimulating agents during the study period.
EndpointsThe endpoints of this study were: a) the relationship between ΔeGFR and changes ΔHb at 1 and 3 months following treatment randomization, and b) the relationship between ΔeGFR and changes in peak-VO2, MLHFQ, and NT-proBNP following treatment with dapagliflozin at both time points.
Statistical analysisContinuous variables are expressed as means (± 1 standard deviation) or medians (interquartile range [IQR]) and discrete variables are expressed as percentages. At baseline, the means, medians, and frequencies among treatment groups were compared using the Student t test, Wilcoxon, and chi-square test, respectively.
Following an ANCOVA design, the main endpoints of this study included absolute (ΔeGFR) and percentage (ΔeGFR%) eGFR changes from baseline. The exposure variables tested for their association with the 2 endpoints were also modeled as changes from baseline and included ΔHb, Δpeak-VO2, ΔMLHFQ, and ΔNT-proBNP. Consequently, all analyses included the baseline value of the endpoint and baseline value of the exposure as covariates. The primary analysis modeled ΔeGFR and the log of ΔHb at 1 and 3 months using linear mixed regression models. In figure 1, values of the log of ΔHb were back-transformed to the raw values by obtaining nonlinear predictions using predictnl command in Stata. Secondary analyses included modeling ΔeGFR independently with Δpeak-VO2, ΔMLHFQ, and ΔNT-proBNP. Each model included patient identification and study center as random intercepts and visits (1 and 3 months) as random coefficients. The variance-covariance structure chosen for the random effects was “unstructured”. The period effect was included by modeling the interaction between the treatment and the period. The covariates included in each model were chosen based on the biological plausibility and regardless of the P value. The list of covariates in each model is presented in the corresponding figure legend and includes the baseline value of eGFR. The results are presented as least square means with 95% confidence intervals (CIs) and P values. The statistical stability of the results was tested with a bootstrap resampling procedure. It was employed based on 300 bootstrap samples (sampling with replacement). All analyses were performed with STATA 16.1 (Stata Statistical Software, Release 16 [2019]; StataCorp United States).
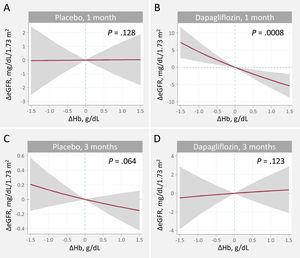
Relationship between hemoglobin and eGFR changes at 1 and 3 months. A. Changes at 1 month, placebo. B. Changes at 1 month, dapagliflozin C. Changes at 3 months, placebo. D. Changes at 3 months, dapagliflozin. ΔeGFR, changes in estimated glomerular filtration rate; ΔHb, changes in hemoglobin.
Estimates adjusted for baseline eGFR, hemoglobin, systolic blood pressure, NT-proBNP, furosemide equivalent doses, treatment with ACEi/ARB/sacubitril-valsartan, and treatment with aldosterone receptor antagonists.
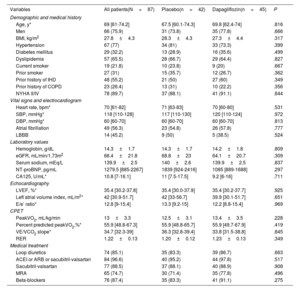
In this analysis, we evaluated 87 patients. The mean age was 67.0±10.5 years, 21 (24.1%) were women, and 72 (82.8%) were stable in NYHA class II. The mean peakVO2, left ventricular ejection fraction, eGFR, and hemoglobin were 13.0±3.3mL/kg/min, 33.7±5.3%, 66.9±20.7mL/min/1.73m2, and 14.3±1.7g/dL, respectively. Thirty (34.5%) and 16 (18.4%) patients showed eGFR ≤ 60 and ≤ 45mL/min/1.73m2 at baseline, respectively. No significant differences in baseline characteristics across treatments were found (table 1), including eGFR and hemoglobin.
Baseline characteristics of the patients stratified by randomization arm
| Variables | All patients(N=87) | Placebo(n=42) | Dapagliflozin(n=45) | P |
|---|---|---|---|---|
| Demographic and medical history | ||||
| Age, y* | 69 [61-74.2] | 67.5 [60.1-74.3] | 69.8 [62.4-74] | .816 |
| Men | 66 (75.9) | 31 (73.8) | 35 (77.8) | .666 |
| BMI, kg/m2 | 27.8±4.3 | 28.3±4.3 | 27.3±4.4 | .317 |
| Hypertension | 67 (77) | 34 (81) | 33 (73.3) | .399 |
| Diabetes mellitus | 29 (32.2) | 13 (28.9) | 16 (35.6) | .499 |
| Dyslipidemia | 57 (65.5) | 28 (66.7) | 29 (64.4) | .827 |
| Current smoker | 19 (21.8) | 10 (23.8) | 9 (20) | .667 |
| Prior smoker | 27 (31) | 15 (35.7) | 12 (26.7) | .362 |
| Prior history of IHD | 48 (55.2) | 21 (50) | 27 (60) | .349 |
| Prior history of COPD | 23 (26.4) | 13 (31) | 10 (22.2) | .356 |
| NYHA II/IV | 78 (89.7) | 37 (88.1) | 41 (91.1) | .644 |
| Vital signs and electrocardiogram | ||||
| Heart rate, bpm* | 70 [61-82] | 71 [63-83] | 70 [60-80] | .531 |
| SBP, mmHg* | 118 [110-128] | 117 [110-130] | 120 [110-124] | .972 |
| DBP, mmHg* | 60 [60-70] | 60 [60-70] | 60 [60-70] | .813 |
| Atrial fibrillation | 49 (56.3) | 23 (54.8) | 26 (57.8) | .777 |
| LBBB | 14 (45.2) | 9 (50) | 5 (38.5) | .524 |
| Laboratory values | ||||
| Hemoglobin, g/dL | 14.3±1.7 | 14.3±1.7 | 14.2±1.8 | .809 |
| eGFR, mL/min/1.73m2 | 66.4±21.8 | 68.8±23 | 64.1±20.7 | .309 |
| Serum sodium, mEq/L | 139.9±2.5 | 140±2.6 | 139.9±2.5 | .837 |
| NT-proBNP, pg/mL | 1279.5 [885-2267] | 1839 [924-2416] | 1085 [889-1688] | .297 |
| CA125, U/mL* | 10.8 [7-16.1] | 11 [7.5-17.5] | 9.2 [6-16] | .711 |
| Echocardiography | ||||
| LVEF, %* | 35.4 [30.2-37.8] | 35.4 [30.0-37.9] | 35.4 [30.2-37.7] | .925 |
| Left atrial volume index, mL/m2* | 42 [30.9-51.7] | 42 [33-56.7] | 39.9 [30.1-51.7] | .651 |
| E/e’ ratio* | 12.8 [9-15.4] | 13.3 [9.2-15] | 12.2 [8.8-15.4] | .969 |
| CPET | ||||
| PeakVO2, mL/kg/min | 13±3.3 | 12.5±3.1 | 13.4±3.5 | .228 |
| Percent predicted peakVO2,%* | 55.9 [48.8-67.3] | 55.9 [48.8-65.7] | 55.9 [49.7-67.9] | .419 |
| VE/VCO2 slope* | 34.7 [32.3-39] | 36.3 [32.8-39.4] | 33.8 [31.5-38.8] | .845 |
| RER | 1.22±0.13 | 1.20±0.12 | 1.23±0.13 | .349 |
| Medical treatment | ||||
| Loop diuretics | 74 (85.1) | 35 (83.3) | 39 (86.7) | .663 |
| ACEI or ARB or sacubitril-valsartan | 84 (96.6) | 40 (95.2) | 44 (97.8) | .517 |
| Sacubitril-valsartan | 77 (88.5) | 37 (88.1) | 40 (88.9) | .908 |
| MRA | 65 (74.7) | 30 (71.4) | 35 (77.8) | .496 |
| Beta-blockers | 76 (87.4) | 35 (83.3) | 41 (91.1) | .275 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CA125, antigen carbohydrate 125; COPD, chronic obstructive pulmonary disease; CPET, cardiopulmonary exercise testing; DBP, diastolic blood pressure eGFR, estimated glomerular filtration rate; IHD, ischemic heart disease; LBBB, left bundle branch block; LVEF: left ventricular ejection fraction assessed by Simpson method; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association functional class; NT-proBNP, N-terminal pro b-type natriuretic peptide: PeakVO2, peak oxygen uptake; RER, respiratory exchange ratio; SBP, systolic blood pressure; VE/VCO2slope, ventilatory efficiency.
Continuous variables are presented as median [interquartile range], unless otherwise specified, and categorical variables as No. (%).
Compared with placebo, eGFR did not significantly change at 1 or 3 months (figure 2). At 1 month, patients allocated to dapagliflozin showed a nonsignificant decrease in eGFR (Δ-2.6mL/min/1.73 m2 [95%CI, -6.8-1.6; P=.163]). At 3 months, the differences continued to be nonsignificant (Δ + 1.9 mL/min/1.73 m2 [95%CI, -2.4-6.2; P=.331]), as shown in figure 1. Likewise, ΔeGFR% did not change across treatment arms at 1 (Δ-2.1% [95%CI,−6.4 to 2.3; P=.495]) or 3 months (Δ + 2.5% [95%CI, −1.9 to 6.9; P=.373]). The proportion of episodes of eGFR decrease >10% from baseline (within changes) was higher in the dapagliflozin group at 1 month (22.2% vs 4.8%; P=.018) but with no differences at 3 months (13.3% vs 16.7%; P=.663). Most patients showing an eGFR decrease> 10% (transient changes) did so at only 1 time point (17 of 21: 80.9%). Only 4 patients showed a persistent eGFR decrease> 10% at 1 and 3 months (persistent changes).
Hemoglobin changesCompared with placebo, hemoglobin significantly increased in patients on dapagliflozin (1-month: Δ + 0.45g/dL [95%CI, 0.03-0.88; P=.037]; and 3-month: Δ + 0.55g/dL [95%CI, 0.12-0.98; P=.012]).
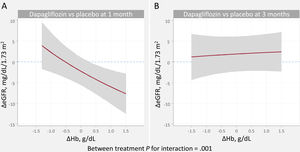
Relationship between changes in estimated glomerular filtration rate and changes in hemoglobinOn multivariate analysis, dapagliflozin treatment differentially affected the association between ΔHb and ΔeGFR across visits (P for interaction=.001). The covariate-adjusted trajectories of ΔHb and ΔeGFR is shown in figure 1. At 1 month, the slope of ΔHb was unrelated to the slope of ΔeGFR in the placebo group (figure 1A, P=.128). Conversely, in patients receiving dapagliflozin, the ΔHb-slope was inversely associated with the ΔGFR-slope (P <.001) (figure 1B). At 3 month, no significant association was found (figure 1C, D). Between-treatment differences (dapagliflozin vs placebo) plots showed that increases in Hb> 0.3g/dL were significantly associated with a significant and almost linear decrease in eGFR at 1 month (95%CIs below the y-line of 0) (figure 3A). At 3 months, we found no between-treatment effect (figure 3B).
Relationship between hemoglobin and eGFR changes. Between-treatment (dapagliflozin vs placebo) comparison. A. Changes at 1 month. B. Changes at 3 months.
ΔeGFR, changes in estimated glomerular filtration rate; ΔHb, changes in hemoglobin.
Estimates adjusted for baseline eGFR, hemoglobin, systolic blood pressure, NT-proBNP, furosemide equivalent doses, treatment with ACEi/ARB/sacubitril-valsartan, and treatment with aldosterone receptor antagonists.
Similar findings were obtained when ΔHb was modeled against ΔeGFR%. A positive ΔHb predicted a decline in ΔeGFR% at 1 month in the dapagliflozin but not in the placebo arm. Compared with placebo, an increase in hemoglobin was associated with a decrease in eGFR in the dapagliflozin group (figure 1 of the supplementary data). At 3 months, there was no relationship between ΔHb and ΔeGFR% with either treatment, with no evidence of a significant difference between treatment effects (figure 2 of the supplementary data).
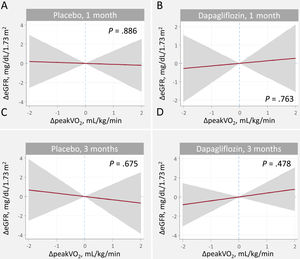
Relationship between changes in estimated glomerular filtration rate and efficacy endpointsChanges in peakVO2We found no evidence of a significant relationship between ΔeGFR and Δpeak-VO2 in either treatment arms or time points (figure 4). Similarly, the between-treatment comparison showed no significant differences (figure 3 of the supplementary data).
Relationship between changes in peakV02 and changes in eGFR at 1 and 3 months. A. Changes at 1 month, placebo B. Changes at 1 month, dapagliflozin. C. Changes at 3 months, placebo. D. Changes at 3 months, dapagliflozin
ΔeGFR, changes in estimated glomerular filtration rate; ΔpeakVO2, changes in peak oxygen consumption.
Estimates adjusted for baseline eGFR, hemoglobin, systolic blood pressure, NT-proBNP, furosemide equivalent doses, treatment with ACEi/ARB/sacubitril-valsartan, and treatment with aldosterone receptor antagonists.
Dapagliflozin and placebo-associated ΔeGFR were not related to ΔMLHFQ or ΔNT-proBNP at either time point (figure 4 of the supplementary data). In addition, between-treatment plots showed no significant differences (figures 5 and 6 of the supplementary data).
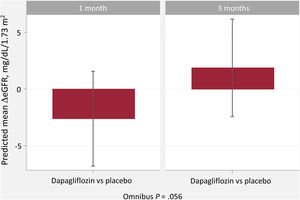
DISCUSSIONIn this post hoc analysis of the DAPA-VO2 trial, we found that 1-month changes in eGFR were inversely related to changes in hemoglobin after dapagliflozin initiation in patients with stable ambulatory HFrEF. In other words, the initial eGFR decline was related to hemoglobin increase, a recognized parameter indicating a favorable clinical response. This association was no longer significant at 3 months (figure 5). Current findings suggest a short-term physiological connection between the kinetics of both markers, suggesting that hemoconcentration may explain part of it. This may also explain the absence of a significant relationship between short-term changes in eGFR and changes in functional ability, quality of life, and natriuretic peptides. At longer follow-up (3 months), changes in eGFR seemed to be unrelated to hemoglobin changes.
Central illustration. Relationship between short-term glomerular filtration rate and hemoglobin changes following dapagliflozin initiation in heart failure with reduced ejection fraction.
eGFR, estimated glomerular filtration rate; HFrEF, heart failure with reduced ejection fraction; NS, not significant.
A significant increase in hemoglobin and hematocrit have been described after SGLT2i initiation.11 Interestingly, hematocrit changes were the strongest single predictor for reducing the risk of cardiovascular death in the EMPA-REG OUTCOME trial.12 More recently, Fitchett et al.13 reported that hemoglobin and hematocrit accounted for more than half of mortality and hospitalization risk reduction attributed to empagliflozin in patients with type 2 diabetes and established cardiovascular disease. In patients with decompensated HF, the relative increase in cellular elements in the blood is commonly used to monitor diuretic response and is associated with improved outcomes. Among them, hemoglobin and hematocrit are the most widely used parameters,14 and are included in the most frequently used formulas for estimating plasma volume.15 The precise mechanism underlying the beneficial outcomes of HF remains uncertain, specifically regarding whether the observed benefits are attributed to a direct rise in hematocrit or are instead mediated by factors which are associated with the increase in hematocrit, although the latter appears to be the more probable explanation.7
There is evidence endorsing the diuretic, predominantly aquaretic, effect of SGLT2i.16,17 Some studies have argued that the increase in hematocrit and hemoglobin levels may be explained by circulating volume contraction, especially in the short-term.18 Thus, we envision hemoconcentration may play a role in explaining the hemoglobin increase, at least in the first days or weeks after SGLT2i onset.7 However, in the mid- to long-term, other factors, such as increased erythropoietin production and changes in iron metabolism,19,20 may explain most of mass changes in red cells.
Pathophysiology of renal function changes after sodium-glucose cotransporter-2 inhibitors initiationPrior studies with SGLT2i in patients with chronic stable HF,21,22 type 2 diabetes,23,24 and chronic kidney disease,25 consistently showed a slight short-term decline in eGFR, followed by a slower decline over time, and a reduction in adverse renal events than in patients on placebo.21–25 Likewise, in a substudy of the EMPULSE trial, Voors et al.26 reported that patients hospitalized for acute HF treated with empagliflozin showed a decline in renal function at 15 days, with no changes at 90 days. In a substudy of the EMPEROR-Reduced Trial, Zannad et al.27 described that a modest decrease in eGFR was observed more frequently in patients receiving empagliflozin than in those receiving placebo. In contrast to placebo-treated patients, these changes were not associated with a worse prognosis. Most of the evidence from controlled studies shows that the initial eGFR dip following SGLT2i initiation is small, transient, and has no deleterious clinical implications. Likewise, this same message has been replicated in large real-world studies in SGLT2i users.28 The findings of the present study are in line with these messages, by showing no relationship between eGFR changes and changes in maximal functional capacity, quality of life, and natriuretic peptides.
The mechanisms behind this initial eGFR decline following SGLT2i initiation are not entirely understood. In type 2 diabetes studies, most of these changes have been attributed to intraglomerular hemodynamic changes (an increase in tubular sodium and chloride excretion is sensed by the macula densa, leading to afferent vasoconstriction, resulting in a reduction in renal blood flow and thereby glomerular filtration).29 However, it may not be the only mechanism under eGFR changes in the whole spectrum of patients with HF, as in most of them, we do not have evidence of hyperfiltration.30
In acute HF, some data show that the clinical significance of creatinine increase/eGFR decrease largely depends on surrogates of decongestion and diuretic response.31,32 For instance, in 2 large cohorts of patients with acute HF, worsening renal function in the first 4days was not associated with worse outcomes when patients had a good diuretic response.31 Interestingly, in a substudy of the EVEREST trial, McCallum, et al.32 reported a heterogeneous effect of eGFR decline across surrogates of decongestion and hemoconcentration. Specifically, the eGFR decline was not associated with adverse outcomes in patients with a greater increase in hematocrit and a decrease in NT-proBNP. In contrast, a decrease in eGFR was associated with worse outcomes in patients with no evidence of decongestion/hemoconcentration.32 Interestingly, in the same study, patients with a greater decrease in renal function were those with a higher increase in hematocrit.32 Thus, in patients with HF, particularly in the acute setting, eGFR changes should be interpreted taking into account their clinical and decongestion status. Small and transient changes in patients with an appropriate diuretic response may be due to hemoconcentration rather than true worsening renal function.33
The clinical significance of the connection between hemoglobin increase and eGFR decline following initiation of SGLT2iIn the HF setting, and according to the results of the current study, we postulated that initial eGFR dip following SGLT2i initiation may reflect hemoconcentration rather than other mechanisms. The following points endorse this hypothesis.
- a)
In patients with HF treated with diuretics, short-term increases in hemoglobin and a decrease in eGFR are recognized proxies of hemoconcentration. For eGFR, this is especially true when the decrease occurs in parallel with evidence of the presence of other parameters of hemoconcentration and adequate clinical and diuretic response.32,33 Along this line of thought, a substudy of the EMPA-RESPONSE showed that empagliflozin increased plasma osmolality and was associated with a temporary decline in eGFR.8 In the present study, we found a strong association between 1 month hemoglobin increase and eGFR decline, as previously found with the use of another aquaretics such as tolvaptan.32 Thus, and given the important role of fluid overload in patients with HF,34 we speculate that in the short-term, most of the beneficial short-term beneficial effects of dapagliflozin may be due to decongestion. At longer follow-up, the kinetics of both biomarkers seem unrelated, suggesting that mechanisms other than decongestion/hemoconcentration may be playing a predominant role.
- b)
The initial eGFR decline after SGLT2i initiation is modest and transient and usually has no clinical consequences. The findings of the present study provide further evidence of this and showed that patients with an initial drop at 1 month showed no eGFR decline at 3 months. Additionally, these changes were modest in magnitude and, importantly, unrelated to functional status or quality of life impairment.
Further confirmatory studies are warranted to confirm these findings in HF and other clinical scenarios, such as chronic kidney disease, in which SGLT2i also has demonstrated clinical usefulness.35,36
LimitationsTo our knowledge, this is the first study that correlates the increase in hemoglobin following SGLT2i administration with changes in renal function. However, the study has some limitations. First, this is a post hoc analysis of a randomized clinical trial. Since our findings were not corrected for multiple testing, there is an increased risk of type I error.37 Second, this study has the inherent limitations of being a trial with limited statistical power. Third, most renal function changes were mild and transient; thus, these findings cannot be extrapolated to more severe forms of renal impairment. Fourth, we cannot explore mid- to long-term changes in kidney function and hemoglobin or infer their biological or clinical significance with the current data. Fifth, hematocrit was not uniformly registered during the trial, precluding evaluation of its relationship with changes in GFR. Sixth, we cannot extrapolate these finding to other clinical scenarios other than HF that frequently use SGLT2i. Finally, we did not measure plasma volume, osmolarity, or other parameters that could help us to support the pathophysiology of this interaction.
CONCLUSIONSIn patients with stable HFrEF, 1-month changes in eGFR induced by dapagliflozin were inversely related to changes in hemoglobin. However, eGFR and hemoglobin changes at 3 months were not associated. Further studies are required to confirm these findings and unravel the biological meaning of this association.
FUNDINGThis work was supported in part by an unrestricted grant from Astra Zeneca (ESR-17-13447), Unidad de Investigación Clínica y Ensayos Clínicos INCLIVA Health Research Institute, Spanish Clinical Research Network (SCReN); grant numbers: PT17/0017/0003 and PT20/00100, and CIBERCV; grant numbers 16/11/00420, 16/11/00403, and 16/11/00486.
AUTHORS’ CONTRIBUTIONSG. Miñana and R. de la Espriella: conceptualization, data curation, investigation, methodology, project administration, validation, visualization, writing—original draft; writing, review and editing. P. Palau, M. Amiguet and J. Seller: data curation, investigation, methodology, validation, visualization, writing, review and editing. J. M. García Pinilla: investigation, methodology, validation, visualization, writing, review and editing. E. Núñez: formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, writing, review and editing. J. L. Górriz, A. Valle, J. Sanchis, and A. Bayés-Genís: investigation, methodology, validation, visualization, writing, review and editing. J. Núñez: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft; writing, review and editing.
CONFLICTS OF INTERESTG. Miñana has received speaker fees from Abbott Vascular; R. de la Espriella reports personal fees from Astra Zeneca, Novartis, Boehringer-Ingelheim, and NovoNordisk; P Palau has received fees for participating in educational activities from Servier; M. Amiguet reports personal fees from Astra Zeneca, Novartis, Boehringer-Ingelheim, Lilly, and Pfizer; J. Seller reports speaker fees from Astra Zeneca and Boehringer-Ingelheim; J.M. García Pinilla reports personal fees from Astra Zeneca and Esteve; J.L. Górriz has received fees for participating in advisory boards and educational activities from Astra Zeneca, Boehringer-Ingelheim, NovoNordisk, Bayer, and Novartis; A. Valle reports speaker fees from Astra Zeneca; J. Sanchis has received speaker fees from Abbott Vascular and Prosmédica; A. Bayés-Genís has lectured and/or participated in advisory boards for Abbott, Astra Zeneca, Boehringer-Ingelheim, Novartis, Roche Diagnostics, and Vifor; J. Núñez reports personal fees from Astra Zeneca, Novartis, Boehringer-Ingelheim, Eli Lilly, Rovi, NovoNordisk, and Vifor Pharma. J. Sanchis is editor-in-chief of Rev Esp Cardiol. The journal's editorial procedure to ensure impartial handling of the manuscript has been followed. The rest of the authors have nothing to declare.
- -
The use of SGLT2i has been associated with a short-term reduction in eGFR.
- -
These changes in eGFR are generally mild, transient, and are not associated with an adverse prognosis.
- -
Another common finding after SGLT2i initiation is a short-term increase in hemoglobin and hematocrit. The magnitude of this increase has been associated with favorable clinical responses mediating the benefit attributable to SGLT2i in patients with type 2 diabetes and HF.
- -
In this subanalysis of the DAPA-VO2 trial, we found that changes in eGFR at 1 month were inversely associated with changes in hemoglobin after dapagliflozin initiation in stable patients with HFrEF.
- -
The association between changes in eGFR and hemoglobin was no longer observed at 3 months.
- -
Our findings suggest a short-term physiological connection between the kinetics of the 2 markers, suggesting that a partial explanation may be the role of hemoconcentration.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.03.007