As the COVID-19 outbreak has increased worldwide, many countries have imposed lockdown restrictions to movement. Since 14 March 2020 in Spain, most people have been confined to home with an absolute ban on outdoor physical activity.
While the number of in-hospital positive COVID-19 patients was growing exponentially, there was a drastic decline in non–COVID-19 emergency patients with a drop of nearly 40% of ST-elevation myocardial infarction patients worldwide.1
Although the number of non–COVID-19 emergency patients decreased, there was an increase in the number of pulmonary embolisms (PE) in non–COVID-19 patients. In this scenario, the role of thromboprophylaxis is uncertain.2
From 14 March to 18 April 2020, in our center we diagnosed 17 acute PE with computed tomography pulmonary angiography. The number of PE cases clear increased compared with 2019 (average of 8 PE cases per month in 2019, with 9 cases from 14 March to 18 April, 2019). To examine whether there was a quarantine-related effect in the increased rate of acute PE, we compared the characteristics of acute PE patients by lockdown subgroups (ie, 14 March to 18 April, 2020) vs the no-lockdown period (ie, 14 March to 18 April, 2019) (table 1) in a single-center observational case series study.
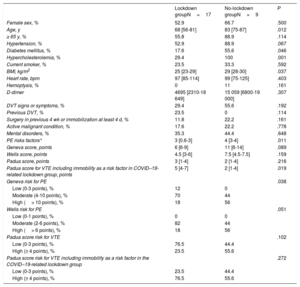
Characteristics of patients with acute pulmonary embolism lockdown subgroups (14 March to 18 April, 2020) vs the no-lockdown period (14 March to 18 April, 2019)
| Lockdown groupN=17 | No-lockdown groupN=9 | P | |
|---|---|---|---|
| Female sex, % | 52.9 | 66.7 | .500 |
| Age, y | 68 [56-81] | 83 [75-87] | .012 |
| ≥ 65 y, % | 55.8 | 88.9 | .114 |
| Hypertension, % | 52.9 | 88.9 | .067 |
| Diabetes mellitus, % | 17.6 | 55.6 | .046 |
| Hypercholesterolemia, % | 29.4 | 100 | .001 |
| Current smoker, % | 23.5 | 33.3 | .592 |
| BMI, kg/m2 | 25 [23-29] | 29 [28-30] | .037 |
| Heart rate, bpm | 97 [85-114] | 99 [75-125] | .403 |
| Hemoptysis, % | 0 | 11 | .161 |
| D-dimer | 4695 [2310-18 649] | 15 059 [6800-19 000] | .307 |
| DVT signs or symptoms, % | 29.4 | 55.6 | .192 |
| Previous DVT, % | 23.5 | 0 | .114 |
| Surgery in previous 4 wk or immobilization at least 4 d, % | 11.8 | 22.2 | .161 |
| Active malignant condition, % | 17.6 | 22.2 | .778 |
| Mental disorders, % | 35.3 | 44.4 | .648 |
| PE risks factors* | 3 [0.6-3] | 4 [3-4] | .011 |
| Geneva score, points | 6 [6-9] | 11 [6-14] | .089 |
| Wells score, points | 4.5 [3-6] | 7.5 [4.5-7.5] | .159 |
| Padua score, points | 3 [1-4] | 2 [1-4] | .216 |
| Padua score for VTE including immobility as a risk factor in COVID–19-related lockdown group, points | 5 [4-7] | 2 [1-4] | .019 |
| Geneva risk for PE | .038 | ||
| Low (0-3 points), % | 12 | 0 | |
| Moderate (4-10 points), % | 70 | 44 | |
| High (> 10 points), % | 18 | 56 | |
| Wells risk for PE | .051 | ||
| Low (0-1 points), % | 0 | 0 | |
| Moderate (2-6 points), % | 82 | 44 | |
| High (> 6 points), % | 18 | 56 | |
| Padua score risk for VTE | .102 | ||
| Low (0-3 points), % | 76.5 | 44.4 | |
| High (≥ 4 points), % | 23.5 | 55.6 | |
| Padua score risk for VTE including immobility as a risk factor in the COVID–19-related lockdown group | .272 | ||
| Low (0-3 points), % | 23.5 | 44.4 | |
| High (≥ 4 points), % | 76.5 | 55.6 |
BMI, body mass Index; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Data are expressed as No. (%), or median [interquartile range].
PE risks factors*: Strong risk factors: fracture of lower limb, hospitalization for heart failure or atrial fibrillation/flutter (within previous 3 months), hip or knee replacement, major trauma, myocardial infarction (within previous 3 months), previous VTE, spinal cord injury. Moderate risk factors: arthroscopic knee surgery, autoimmune diseases, blood transfusion, central venous lines, intravenous catheters and leads, chemotherapy, congestive heart failure or respiratory failure, erythropoiesis-stimulating agents, hormone replacement therapy, in vitro fertilization, oral contraceptive therapy, postpartum period, infection (specifically pneumonia, urinary tract infection, and human immunodeficiency virus, inflammatory bowel disease, cancer (highest risk in metastatic disease), paralytic stroke, superficial vein thrombosis, and thrombophilia. Weak risk factors: bed rest> 3 days, diabetes mellitus, hypertension, immobility due to sitting, increasing age, laparoscopic surgery, obesity, pregnancy, and varicose veins.
Comparison between categorical data was performed using the chi-square test or the McNemar test for paired data and the Mann-Whitney U-test for ordinal and continuous variables. Statistical analysis was performed with SPSS version 21 (SPSS Inc, Chicago, IL) and a value of P <.05 was considered the threshold for statistical significance.
Patients in the PE lockdown period were younger (median age, 68 years; interquartile range [IQR][56-81] versus 83 [75-87] years; P=.012), with a lower prevalence of diabetes mellitus (17.6% vs 55.6%; P=.046), hypercholesterolemia (29.4% vs 100%; P=.001), and lower body mass index) (median body mass index=25 [23-29] vs 29 [28-30]; P=.037).
There were numerous environmental and patient-related predisposing venous thromboembolism (VTE) risk factors that we summarize in table 1, as described in the European Society of Cardiology guidelines for acute PE.3
Patients in the COVID–19-related lockdown period had a lower number of PE risk factors (median PE risk factors, 3 [0.6-3] vs 4 [3-4]; P=.011) (table 1). COVID–19-related lockdown patients also had a significantly lower PE risk when assessed with the Wells and Geneva risk scores as categorical (low, moderate, and high risk) variables.
Sixteen patients had VTE risk factors added to prolonged immobility due to quarantine; 11 patients had moderate or strong risk factors for PE (table 2). Only 1 patient with chronic lymphocytic leukemia had a positive nasal-pharyngeal swab sample polymerase chain reaction (PCR) for COVID-19 at diagnosis. One patient, who had previous COVID-19 severe pneumonia and negative nasal-pharyngeal swab sample at discharge, developed acute PE 1 week later.
Patients with acute pulmonary embolism during the COVID–19-related lockdown period (14 March to 18 Abril, 2020) and during the no-lockdown period (14 March to 18 Abril, 2019)
| Year | Sex | Age | BMI | Smoker | HTA | Hypercholesterolemia | Diabetes | Mental disorder | PE risk factors | COVID-19 | d-dimer | RV dysfunction | DVTDoppler US | DVTSigns/symptoms | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | Female | 73 | 34 | Yes | Yes | No | No | No | -Active lung cancer-Obesity | - | - | Yes | - | Yes | Yes |
| 2019 | Female | 87 | 29 | No | Yes | Yes | Yes | Anxiety | -Reduced mobility-Overweight | - | 17 051 | No | Yes | Yes | No |
| 2019 | Male | 44 | 28 | No | Yes | Yes | Yes | No | -Rheumatoid arthritis under treatment-Overweight | - | 7485 | No | Yes | Yes | No |
| 2019 | Female | 86 | 29 | No | Yes | Yes | No | No | -Overweight-Advanced age | - | 15 059 | No | Yes | Yes | No |
| 2019 | Male | 87 | 30 | Former smoker | Yes | Yes | No | No | -Vertebral fracture-Obesity | - | 1474 | No | - | No | Yes |
| 2019 | Female | 84 | 31 | Yes | Yes | Yes | Yes | No | -Hip fracture-Obesity | - | - | Yes | - | No | |
| 2019 | Female | 75 | 32 | Yes | Yes | Yes | Yes | ParkinsonAnxiety | - Obesity | - | 6800 | No | No | No | No |
| 2019 | Male | 76 | 30 | No | No | Yes | Yes | Bipolar disorder | -No active colorectal cancer-Obesity | - | 19 000 | Yes | Yes | No | No |
| 2019 | Female | 83 | 25 | No | Yes | Yes | No | Psychotic disorderDementia | - Active colorectal cancer | - | 71 649 | No | - | Yes | No |
| 2020 | Male | 56 | 32 | No | Yes | Yes | No | Anxiety | -DVT under LMWH treatment-Obesity | No | 1117 | No | Yes | Yes | No |
| 2020 | Female | 69 | 24 | Yes | No | No | No | No | -Previous PE with DVT | No | 2800 | No | Yes | Yes | No |
| 2020 | Female | 43 | 23 | No | No | No | No | No | -Oral contraceptive | No | 4695 | No | No | No | No |
| 2020 | Female | 34 | 25 | No | No | No | No | Psychotic disorders | -Psychotic attack-Bedridden-Obesity | No | 63 409 | Yes | No | No | No |
| 2020 | Male | 56 | 33 | No | No | No | No | No | -Previous DVT-Obesity | No | 2639 | No | Yes | Yes | No |
| 2020 | Male | 62 | 17 | Yes | No | No | No | No | -Orchiepididymitis-Bedridden | No | - | Yes | No | No | No |
| 2020 | Male | 83 | 21 | Former smoker | No | No | No | No | -Advanced age | No | 18 649 | Yes | - | No | No |
| 2020 | Female | 56 | 23 | Yes | No | Yes | No | Psychotic disorders | No | No | 27 361 | No | Yes | No | No |
| 2020 | Male | 81 | 28 | No | No | Yes | No | -Chronic lymphocytic leukemia | Positive | 6057 | No | - | No | No | |
| 2020 | Female | 70 | 29 | No | Yes | No | No | Depression | -Overweight | No | 3685 | No | No | No | No |
| 2020 | Female | 68 | 30 | No | Yes | No | Yes | No | -Active breast cancer-Obesity | No | 2006 | No | - | No | No |
| 2020 | Male | 67 | 28 | No | Yes | No | No | No | -Previous PE with DVT | No | 15 252 | Yes | No | No | No |
| 2020 | Male | 85 | 29 | No | Yes | No | No | No | -Advanced age-Overweight | No | 2310 | Yes | No | No | Yes |
| 2020 | Female | 70 | 17 | No | Yes | Yes | No | No | -Ventricular dysfunction with heart failure | No | 800 | Yes | No | No | No |
| 2020 | Female | 83 | 23 | No | Yes | Yes | Yes | Dementia | -COVID-19 infection discharged with negative PCR-Advanced age | Discharged for COVID-19 infection 1 week before | 13 340 | No | - | No | No |
| 2020 | Female | 71 | 32 | No | No | Yes | No | Parkinson's - Dementia | -Parkinson's Dementia disease-Obesity | No | - | Yes | - | No | No |
| 2020 | Male | 65 | 25 | Yes | Yes | No | No | No | Active pancreatic cancer under LMWH treatment | No | 137 741 | No | - | No | No |
BMI, body mass index; DVT, deep vein thrombosis; LMWH, low molecular weight heparin; PCR, polymerase chain reaction; PE, pulmonary embolism; PHT, pulmonary hypertension; RV dysfunction, right ventricular dysfunction.
When asked about previous daily activity, most patients reported a previously active lifestyle followed by a sedentary lifestyle during the quarantine with prolonged immobility.
Six patients had mental disorders that could worsen immobility during the quarantine and predispose them to PE,4 but we found no significant difference between the groups corresponding to the COVID-19-related lockdown period and the non–COVID-19 period in the prevalence of mental disorders (35.3% vs 44.4%; P=.648).
In an attempt to explain that immobility due to the hard lockdown could be one of the triggers for PE, we calculated the Padua score, which stratifies patients as being at high (≥ 4 points) or low (< 4 points) risk for VTE. We considered the hard lockdown quarantine as a “reduced mobility” risk factor; immobility in this score is penalized with 3 points. There was no significant difference in the baseline Padua prediction score for VTE (median Padua score, 3 [1-4] vs 2 [1-4]; P=.216). The COVID–19-related lockdown group had a significantly higher score in the subanalysis including immobility as a risk factor during the lockdown (median Padua score, 5 [4-7] vs 2 [1-4]; P=.019). We found a significant increase in high-risk patients in the lockdown subgroup considering lockdown as immobility (Padua score without immobility: 23.5% patients at high risk, Padua score with immobility: 76.5% patients at high risk; P=.004). When we compared the Padua score as a categorical risk variable, we found no significant difference between the lockdown period group and the no-lockdown group.
We hypothesized that a rigorous quarantine in patients with strong risk factors could predispose them to acute PE. Immobility causes an 6-fold increase in the risk of deep vein thrombosis (or PE in patients with previous events compared with patients without deep vein thrombosis or PE history).5
The increasing number of COVID–19-related acute PE cases described recently suggests that COVID-19 infection could be an added risk factor for acute PE during quarantine. In our series, the low prevalence of COVID-19 infection on nasal smear PCR tests does not suggest a causative relationship. A single effect, either of quarantine immobility or undiagnosed COVID-19 infection, cannot be excluded and would require a large study including COVID-19 serology-based testing with high sensitivity and specificity.
In the emergency department, elevated D-dimer with dyspnea in COVID-19 quarantine patients might be misleading. Clinicians should pay attention to a possible PE in the setting of a COVID-19 infection.
In nations imposing a hard lockdown, all patients with VTE risk factors might be counseled for mechanical prophylaxis and to stay active at home. Pharmacological prophylaxis could be advised in patients at high risk, especially previous VTE and active malignancy, which must be weighed against the risk of bleeding.
.