In recent years, the number of heart transplants has stabilized, leading to widespread use of ventricular assist devices as a destination therapy.1
We present the case of a 72-year-old woman who presented to our center with a 6-hour history of chest pain and electrocardiographic changes consistent with anterior acute myocardial infarction. Emergent coronary angiography showed thrombotic occlusion of the proximal left anterior descending artery with damage to the distal and proximal circumflex branch. Complete percutaneous revascularization was therefore performed with 3 drug-eluting stents. The patient developed cardiogenic shock, requiring support with vasoactive amines and an intra-aortic balloon pump during the acute phase. She was stabilized and discharged from hospital 3 weeks later. In the 3 months that followed, the patient's clinical course was unfavorable, requiring hospital admission on various occasions due to heart failure, and the use of intravenous inotropic agents. The echocardiogram and cardiac magnetic resonance showed adverse remodeling, severe ventricular dysfunction, and severe functional mitral regurgitation. Cardiorespiratory study showed peak oxygen consumption of 11.4mL/kg/min (Weber class C) and the hemodynamic study showed a heart rate of 1.8 l/min/m2 and a pulmonary capillary pressure of 27 mmHg. With an INTERMACS score of 3, the decision was made to implant a left ventricular assist device as a destination therapy, and the HVAD device (HeartWare Inc.; Miami Lakes, United States) was chosen due to the patient's small body surface area. Surgery was minimally invasive with an upper hemisternotomy approach combined with left anterior minithoracotomy. Following implantation, the patient showed a gradual, sustained improvement.
Monitoring of the HVAD device was carried out by means of transoesophageal echocardiogram in the initial phases and transthoracic echocardiogram thereafter, limited by the absence of an apical acoustic window due to the position of the device. A comprehensive assessment was chosen (vascular, cardiac and assist device) using 4-dimensional cardiac computed tomography (CT).
This was performed using Brilliance iCT-256 equipment (Philips Healthcare; The Netherlands), retrospective spiral chest coverage (100 keV, 550 mA, modulated at 40 and 78% of the cycle; estimated effective dose 5.4 mSv), bolus tracking in the mid descending aorta (threshold 150 HU; delay after threshold 7 s), 3-phase injection using iodixanol 320 mg/mL in the following sequence: a) 60 mL of contrast at 5 mL/s; b) 30 mL of contrast at 3 mL/s, and c) 40 mL of saline solution at 3 mL/s. The images were reconstructed every 5% of the cardiac cycle (iDose4 iterative reconstruction, hard kernel; FOV 512 × 512 mm; final resolution 0.4 × 0.5 × 0.5 mm; 20 Hz). Postprocessing was carried out with specific software (Comp Cardiac; Philips Healthcare, The Netherlands). After reconstruction of the longitudinal cardiac axes and the short axis, the end-diastolic and end-systolic endocardial borders of both ventricles were traced for calculation using the Simpson method (Figure 1). We confirmed the correct position of the device and the integrity of the anastomoses, and ruled out improper septum or papillary suction, thrombosis in the suction cone or ejection cannula (Figure 2 and Video of the supplementary material). Both ventricles were shown to be of normal size, with extensive akinesis in the anterior territory. The left ejection fraction was 34% (ejection volume 36mL) and the right ejection fraction was 54% (ejection volume 88mL). Given that it was not possible to measure flows with cardiac CT, in the absence of an intracardiac shunt, with minimal aortic valve opening and having ruled out significant valvular regurgitation in previous echocardiograms, we indirectly estimated a device support of 3.1 l/min.
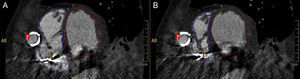
Postprocessing of the biventricular systolic function analysis. End-diastolic (A) and end-systolic (B) endocardial contours manually traced in the short axis covering both ventricles. Ejection cannula (#) making an impression on the lateral wall of the right ventricle. Electrode from implantable cardioverter defibrillator (+).
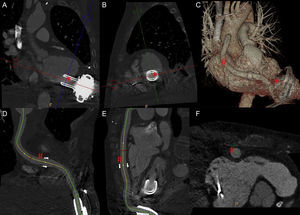
Multiplanar reconstruction of the HVAD suction cone (*) in the longitudinal axis (A) and transverse axis (B). Three-dimensional volumetric reconstruction of the ventricular assist device (C). Curved multiplanar reconstruction of the ejection cannula (#) in longitudinal planes (D and E) and transverse planes (F).
The HVAD device consists of a third-generation continuous flow centrifugal pump which, given its small size (approximately 50 mL), allows direct apical implantation in the pericardial space and an anastomosed ejection cannula directly in the ascending aorta. Right ventricular dysfunction is the main prognostic factor and has been identified in as many as 30% of patients after implantation. The geometric change in the interventricular septum following unloading of the left ventricle or through direct suction from the device, as well as an increase in systemic venous return in the support situation, can reveal silent right systolic dysfunction.2
Monitoring by means of imaging techniques with echocardiogram is recommended and cardiac CT should be considered for specific cases with incomplete information.3 The sensitivity and specificity of cardiac CT for detecting thrombi or poor position are 85% and 100%, respectively.4 Previous series have demonstrated the reproducibility of the right systolic function calculation in the monitoring of ventricular assist devices using cardiac CT, although the majority were second-generation devices.5
Our case is of interest as it is, to the best of our knowledge, the first to describe HVAD with comprehensive assessment by means of 4-dimensional cardiac CT with more than 64 detectors.