Postoperative spasm localized to native or grafted vessels is observed in coronary surgery in up to 11% of patients and usually resolves spontaneously or after the administration of vasodilators.1,2 In contrast, diffuse spasm affects 0.8% to 1.3%, is difficult to treat, and is associated with a high mortality.1,2 The mechanisms associated with postoperative spasm include the administration of vasoconstrictors and beta-blockers, iatrogenic damage due to vessel handling or CO2 insufflation, autacoid-mediated vasoconstriction, hypothermia, hypomagnesemia, smoking, diabetes mellitus, and ischemia-reperfusion damage.1–3 Diffuse spasm usually occurs at the end of the intervention or in the immediate postoperative period and manifests as ST-segment elevation, ventricular arrhythmias, hypotension, and eventually heart and circulatory failure.1,2
The treatment for diffuse spasm involves vasodilator administration and the use of a counterpulsation balloon.1–3 However, these measures may be insufficient, with rapid progression of cardiogenic shock.1,2
Circulatory support with venoarterial extracorporeal membrane oxygenator (VA-ECMO) has shown promising outcomes in diffuse spasm refractory to conventional treatment.1,3 We present a case of postoperative diffuse coronary spasm requiring VA-ECMO and Impella device implantation.
A 62-year-old man, a smoker, with no other past medical history of note, with preserved ventricular function, was referred for surgery for a left main stem lesion. He received extracorporeal circulation with hyperkalemic cold-blood cardioplegia, and a double coronary artery bypass graft with the left mammary grafted to the anterior interventricular artery and a right mammary Y-graft to the first marginal branch. The extracorporeal circulation was disconnected without any events, flow was 36mL/min through the anterior interventricular graft and 28mL/min through the marginal, with pulsatility indexes of 1.9 and 2.1, respectively.
Immediately after sternal closure, the patient developed ST-segment elevation and hypotension, so the chest was reopened and the patency of the grafts was confirmed. After a few minutes and after administration of inotropes, vasoconstrictors and volume expanders, electrocardiographic and hemodynamic normality was recovered. The episodes of transient ST-elevation accompanied by hypotension occurred several times over a period of about 15minutes, so a counterpulsation balloon was inserted via femoral puncture. Despite this, the patient had progressive hemodynamic deterioration (hypotension and biventricular dilatation) with persistent ST elevation, so a VA-ECMO was implanted via femoral access (Cardiohel Maquet Cardiopulmonary, Hirlinge, Germany). The ECMO provided hemodynamic stability and allowed the chest to be closed, but the QRS widening, runs of ventricular tachycardia, and bradycardia persisted.
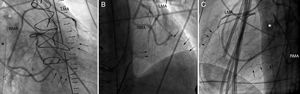
The patient was then transferred to the catheterization laboratory, where coronary angiography showed diffuse spasm throughout the left coronary tree, more pronounced in the distal segments, with patent mammary grafts (Figure 1). The spasm did not resolve despite intracoronary nitroglycerin administration, so a stent was implanted in the left main stem, but no significant angiographic improvement was observed.
Postoperative angiogram: left oblique (A), posteroanterior (B), and lateral (C). Patent RMA and LMA grafts and diffuse spasm of the coronary tree distal to the anastomoses (arrows) can be seen. Extracorporeal membrane oxygenator venous cannula (asterisk). RMA, right mammary artery graft; LMA, left mammary artery graft.
The patient was transferred to the postanesthesia care unit, and for the following 6hours remained on ECMO at 3.8-4 L/min, with good urine output and systolic pulmonary pressures of around 60 mmHg. Transesophageal echocardiography showed a distended, akinetic left ventricle unable to open the aortic valve.
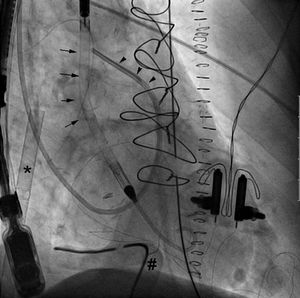
It was decided to unload the left ventricle by implanting an Impella CP via femoral puncture, after removing the counterpulsation balloon (Figure 2). In simultaneous mode, a flow was achieved of 3.8-4.2 L/min with ECMO and 1.2-1.9 L/min with the Impella; adequate left ventricular emptying was observed and pulmonary arterial pressure decreased. Lactate was reduced from 9.41 to 2.06 mmol/L at 8hours after Impella implantation. Peak troponin I was 320 ng/mL on the first day postoperatively.
Assistance with Impella was gradually reduced after 72hours and after confirmation of an improved left ventricular function. The Impella and ECMO were removed surgically after 96hours and 6 days, respectively. The patient progressed satisfactorily, but still had moderate systolic dysfunction at discharge.
VA-ECMO is a safe and effective alternative treatment for coronary spasm, as it provides an adequate output, although it can be insufficient to unload the left ventricle. Among the measures to facilitate ventricular emptying and avoid distension of cardiac chambers are balloon counterpulsation (which was ineffective in our patient), percutaneous atrial septostomy, central cannulation for ECMO, and Impella implantation.4,5 Recently it has been observed that the combined use of ECMO and Impella can provide better outcomes than ECMO alone,6 although it must be remembered that both techniques are invasive and not free from thrombotic or hemorrhagic vascular complications. The case presented here describes for the first time the combined use of ECMO and Impella in cardiogenic shock secondary to postoperative refractory coronary spasm.
.