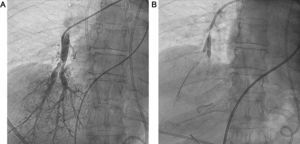
Chronic thromboembolic pulmonary hypertension (CTEPH) is caused by recurrent, unresolved pulmonary embolisms. The thrombi form intraluminal walls and membranes that replace the normal intima of the pulmonary arteries and cause obstruction. Pulmonary thromboendarterectomy is the treatment of choice and offers the only potential cure for CTEPH.1 However, almost 40% of patients with CTEPH are inoperable, due to the location of the peripheral thrombus and/or comorbidities.
Patients who are not candidates for pulmonary thromboendarterectomy are prescribed specific medication for pulmonary hypertension, but many of them have persistent poor functional and hemodynamic status, despite medical treatment. For these patients, balloon pulmonary angioplasty (BPA) has been suggested as a coadjuvant therapy in recent years (Figure).
Since 1996, we have treated 188 patients with CTEPH at our unit, 100 of whom received pulmonary thromboendarterectomy and 88 medical treatment.2 In May 2013, we started performing BPA as coadjuvant therapy in patients with CTEPH who were not candidates for surgery because of distal location or poor clinical and/or hemodynamic status, despite optimized medical treatment. This case series describes our experience of using BPA. To our knowledge, this is the first such case series published in Spain.
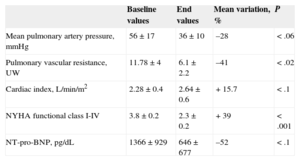
We performed 22 BPAs in 7 patients (5 women; mean age, 61 years), all of whom had New York Heart Association (NYHA) functional class III-IV, despite receiving triple-combination therapy, which included systemic prostanoids in 6 patients. A multidisciplinary team confirmed inoperability and then made a joint decision to perform BPA. A mean of 3 procedures was performed per patient and each procedure involved treatment to a mean of 2.4 segments and 1.2 lobes. In 6 patients, there was significant hemodynamic improvement during follow-up (mean, 6 months [range, 1-18 months]), with a decrease in mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance, and an increase in cardiac index (CI). In addition, right ventricular wall stress decreased, leading to lower N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels, and improved NYHA functional class in all patients. We were able to discontinue prostanoid treatment in 3 out of 6 patients. These data are shown in the Table, and in Tables 1 and 2 of the supplementary material. Two patients had acute reperfusion pulmonary edema as a complication after their first BPA procedure. In 1 patient, the episode was subclinical and was managed with diuretics. The other patient required mechanical ventilation and circulatory support with venoarterial extracorporeal membrane oxygenation (ECMO). She died 8 days post-BPA from a brain hemorrhage (Figures 1 and 2 of the supplementary material). There were no BPA complications involving pulmonary arterial rupture.
Hemodynamic Parameters, Functional Class and Biomarkers at Baseline and After Balloon Pulmonary Angioplasty Procedures
| Baseline values | End values | Mean variation, % | P | |
|---|---|---|---|---|
| Mean pulmonary artery pressure, mmHg | 56±17 | 36±10 | –28 | <.06 |
| Pulmonary vascular resistance, UW | 11.78±4 | 6.1±2.2 | –41 | <.02 |
| Cardiac index, L/min/m2 | 2.28±0.4 | 2.64±0.6 | +15.7 | <.1 |
| NYHA functional class I-IV | 3.8±0.2 | 2.3±0.2 | +39 | <.001 |
| NT-pro-BNP, pg/dL | 1366±929 | 646±677 | –52 | <.1 |
NT-pro-BNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
In 2001, Feinstein et al3 demonstrated improved hemodynamics and exercise tolerance after performing BPA in 18 patients with inoperable CTEPH, although 11 had post-BPA acute reperfusion pulmonary edema and 1 patient died as a result. However, despite these findings, the technique was not widely accepted as alternative or coadjuvant therapy in selected patients with CTEPH until 3 years ago, after the publication of some case series, most of which were Japanese.4 In some series, the technique has been refined by using intravascular ultrasound or optical coherence tomography and, more importantly, by treating only 1 or 2 segments per session, which reduces the onset of acute reperfusion pulmonary edema. Published hemodynamic results show a decrease of as much as 47% in mPAP and of 65% in pulmonary vascular resistance. In our series, we achieved a mean decrease of 28% in mPAP and of 41% in pulmonary vascular resistance, with unequal distribution among treated patients. The improvement obtained with BPA is similar to that achieved with pulmonary thromboendarterectomy (42% reduction in mPAP and 64% reduction in pulmonary vascular resistance) and is significantly better than the reported outcome of medical treatment, with a 9% reduction in mPAP and a 29% reduction in pulmonary vascular resistance.5 Acute reperfusion pulmonary edema is the commonest complication of BPA, and the leading cause of death (1.4%–10%). This complication has a high subclinical incidence of 60%, but mechanical ventilation is required in only 6% of cases. Variables showing a high correlation with the onset of acute reperfusion pulmonary edema are the number of lobes and segments treated per procedure, pre-BPA mPAP>35mmHg, and poor clinical and hemodynamic status preprocedure. The patient in our series who died from acute reperfusion pulmonary edema had NYHA class IV, despite receiving triple-combination therapy with systemic prostanoids. She had a poor hemodynamic profile, with a CI of 2.02 L/min/m2 and a pre-BPA mPAP of 62mmHg, and only 2 segments were treated in a single lobe. The other complication associated with BPA is pulmonary artery wall perforation or rupture, which is a life-threatening, albeit rare, event.
In our experience, and in agreement with the literature, we can confirm that BPA is an effective therapeutic alternative in selected patients with inoperable CTEPH, because it improves hemodynamics, functional capacity, and biomarkers and reduces the need for prostanoid therapy. However, because of the significant incidence of serious periprocedural complications, BPA should be used appropriately and in carefully selected patients.