Keywords
INTRODUCTION
Ever since Ross proposed replacing the aortic valve with the patient's own pulmonary valve (autograft) and the pulmonary valve with a homograft,1 this procedure has proved to be a valuable alternative to aortic valvular replacement in children and young adults.2 The pulmonary autograft can grow,3 has a lower risk of infection,4,5 does not produce haemolysis or require anticoagulation4 and has an excellent haemodynamic profile that allows for the reversal of remodeling.6
Autograft valve failure (AGF) is a possible complication during follow-up. Most series report rates of autograft re-operation,5,7-11 however, echocardiographic studies on the emergence of AGF are scarce4,12,13 and not all of them report rates for this complication. Our goal is to analyse the incidence of AFG during follow-up in patients who have undergone the Ross operation in our institution and to study the factors that predict AFG.
METHOD
From the 102 patients treated with the Ross technique in our hospital from November 1997 to January 2009, we selected those with no significant autograft regurgitation at discharge and with at least one follow-up echocardiogram.
All procedures were elective. The autograft was implanted as a total aortic root replacement. Prior to surgery, a complete echocardiographic evaluation (Acuson Sequoia, Siemens equipment) was performed to analyse ventricular size and function, aortic and pulmonary valve anatomy and function and associated lesions. Since the series included paediatric patients, echocardiographic dimensions were normalised by dividing them by body surface area. We conducted a clinical and echocardiographic follow-up every 6 months for the first year and once a year thereafter.
AGF was defined as the moderate to severe regurgitation of the autograft according to the integrated assessment proposed by the American Society of Echocardiography guidelines,14 whose criteria for considering regurgitation as moderate or severe are, among others: a regurgitation jet width that is ≥25% or ≥65% of the left ventricular outflow tract; a vena contracta ≥0.3 cm2 or >0.6 cm2; a diastolic reversal flow in the descending aorta that lasts longer than the proto-diastolic or holo-diastolic and pressure half-times <500 ms or <200 ms, respectively. The regurgitant volume and fraction and the effective regurgitant orifice area were calculated only in uncertain cases. In case of discrepancies in severity when using the different methods, the ones with the highest quality data were favoured. Endocarditis and surgical pseudoaneurysms cases were excluded.
Comparisons of baseline characteristics between groups with and without AGF were performed using the Student t or the Mann-Whitney U tests, as appropriate, for quantitative variables and the χ2 test for qualitative variables. AGF-free survival was analysed using a Kaplan-Meier curve. A multivariate analysis was performed using the Cox proportional hazards model and we included as covariables all those that in the univariate analysis had shown an association with AGF with P<.1. P values <.05 were considered significant. We used SPSS version 12.0 for the statistical analysis.
RESULTS
From the 102 patients who underwent Ross operations at our centre between November 1997 and January 2009, 19 were excluded from the study: one for early death, 2 for autograft surgery because of pseudoaneurysms (1 for early endocarditis and 1 for iatrogenic cause, both with severe regurgitation), 1 for moderate autograft regurgitation at discharge and 15 for not having a follow-up echocardiogram. In the end, 83 patients (average age, 32 [11]; range, 6-54 years; 60 males [72%]) met the inclusion criteria and constituted the study group.
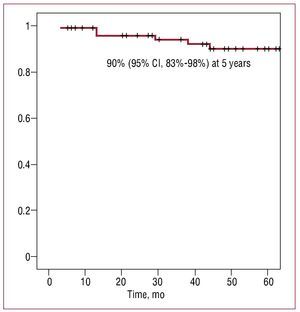
Table 1 shows the baseline characteristics of the 83 patients. After a median (interval) of 4.2 (0.2-10.9) years of follow-up, 8 (9.6%) patients had AGF: 4 with severe failure (3 required valve replacement), 1 with moderate-severe failure and 3 with moderate failure. The probability of AGF-free survival was 90% (95% [CI], 83%-98%) at 5 years (Figure). The rate of autograft reoperation for AGF was 0.79/100 patients/year. In the univariate analysis (Table 2), AGF was associated with the intervention in the first 6 months after starting the implementation of the technique (learning curve), non-congenital aetiology, lower ejection fraction and larger pulmonary ring (normalised by body surface area) in the echocardiogram prior to surgery. In the multivariate analysis, only intervention in the first six months after starting the implementation of the technique (learning curve, HR=9.1; 95% CI, 1.4-59.4; P=.021) and larger normalised pulmonary ring (HR=1.4; 95% CI, 1.016-1.924; P=.04) were independent predictors of AGF, after adjusting for the rest of the variables that were associated with this complication in the univariate analysis.
Figure Survival free of moderate or severe autograft regurgitation.
DISCUSSION
In our series, we noted an autograft reoperation rate for AGF of 0.79/100 patients/year, to the extent described in previous series,7-11 and an AGF rate of 9.6% after 4.2 years of follow-up, somewhat lower than the approximately 14% at 4.4 years reported by other authors.4 Unlike previous studies, we did not observe any influence of age,10 sex,12 aetiology,10 or type of lesion in the appearance of AGF.10,12
One of the main contributions of our study is the impact of the learning curve in the frequency of AGF during follow-up. Beyond that period, the incidence of AGF is extremely low (4%). As expected given the complexity of the Ross operation, the learning curve influenced the results, a fact scarely mentioned in the literature and that needs to be evaluated by centres that employ this technique. Another interesting observation is that larger pulmonary ring size measured prior to surgery, is associated with AGF. This finding is consistent with earlier studies indicating that patients who underwent autograft reoperation had a larger ring initially.12 A possible explanation for this finding, among others, could be that the parietal stress borne by the autograft will be greater the larger its radius, according to Laplace's law. Further studies are needed before recommending a pulmonary ring size beyond which intervention is not advisable.
Our findings indicate that the Ross procedure, when implemented in experienced centres, remains an excellent alternative to conventional aortic valve replacement in certain patients (paediatric, young adults, women of childbearing age). Longer follow-up periods and comparative studies with other alternatives are needed to definitively establish the role of this procedure in the treatment of aortic valve disease in this population.
A limitation of this study is the exclusion of 15% of patients treated in our centre in which no follow-up echocardiography was performed. Other series also suffer from this weakness,13 but despite this, we believe our results help shed light on the clinical evolution of the autograft at 5 years, the incidence of significant failure of the autograft and the value of echocardiography in patient monitoring.
Correspondence: Dr. M. Ruiz Ortiz.
San Adolfo, 18, 3.o A. 14005 Córdoba. Spain
E-mail: maruor@gmail.com
Received June 29, 2009.
Accepted for publication September 16, 2009.