Keywords
INTRODUCTION
Atherosclerosis is a progressive disease characterized by the accumulation of lipids and fibrous elements in arterial walls. One of the most widespread clinical techniques for its assessment is vascular ultrasound examination. This typically consists of the acquisition of B mode ultrasound images of the main arteries in order to look for lesions. It has been shown that the presence of carotid plaques in asymptomatic patients has a positive predictive power of cardiovascular risk greater than that provided by other non-invasive measurements, such as the thickness of the intima-media, the pulse wave velocity or endothelial function.1
The development of atheromatous plaques can be divided into 2 stages; initiation and progression. The risk factors that condition the appearance of these plaques in a patient can be studied, and once a lesion has been detected, the study of its progress can help predict the vulnerability of the patient.2 This vulnerability refers to the probability of the plaque rupturing and the risk of the patient suffering an important cardiovascular event. The factors identified that condition such ruptures include size3,4 (degree of stenosis), the cholesterol concentration,5 and traditional risk factors such as sex, age and smoking.6-8 An additional challenge is to be able to estimate the vulnerability of the lesion early, thus providing information that might help in taking decisions in cases of atherectomy4,9 and prosthetic implantations.10
A number of qualitative methods were initially proposed to characterize atheromatous plaques, but their reproducibility was not always very good.11-13 Quantitative methods then became available which tried to reduce observer dependence.14,15 The traditional technique in this area proposed the calculation of the gray scale median (GSM) of all the pixels represented in a plaque. It is relatively well accepted that dark, homogeneous plaques are an indication of the presence of lipids and therefore that the lesion is vulnerable. Brighter plaques are associated with the presence of calcium, which helps to stabilize them and reduce their vulnerability.16-18 These observations have been verified in symptomatic patients19 and have been compared with histopathological findings.20-22 Factors such as the gain of the ultrasound scan, the image quality, the cut plane of the projection in 2D, and the arbitrariness of the plaque boundaries have all been identified as sources of methodological variation.14,15
The traditional method averages the composition of the atheromatous plaque, such that a single echolucency value is provided. This method does not, therefore, take into account regional variations. Nonetheless, these lesions are generally heterogeneous, and patterns related to the presence of internal hemorrhaging23 or lipid nuclei along with fine but denser surface layers might be associated with high vulnerability.3,16
The aim of the present work was to assess a new strategy for evaluating the composition of atheromatous plaques. This consists of analyzing the GSM of the plaque stratified in layers parallel to the surface. Each plaque is thus characterized by its layers via a GSM curve with respect to depth, from the lumen towards the adventitia. The reproducibility of the traditional model and the proposed "bilayer" model is discussed, along with the influence of certain risk factors on echogenicity curves.
METHODS
Study Subjects
The study subjects were 42 asymptomatic patients who were attended to at the Centre de Médicine Préventive Cardiovasculaire of the Georges Pompidou Hospital in Paris (France). All atheroma plaques were recorded by the same operator. All patients gave their signed consent to be included in the study, thus meeting the ethical requirements of the institution.
Acquisition and Quantification of Images
Femoral and carotid arteries were visualized longitudinally using a high resolution ultrasound apparatus (Logic 9, General Electric) in B mode (transducers 9 MHz). The images were digitalized using a computer (Pentium 4, 1 GHz, 512 MB RAM (random access memory), hard disk 120 GB) equipped with a Data Translation DT3130 series data capture card (Marlboro, Maryland, USA). Image resolution was fixed at 768 ´ 576 pixels and 256 scales of gray (0=black; 255=white). Image processing and plaque quantification was performed using a Hemodyn 4M apparatus (Dinap SRL, Argentina). Image quantification was always performed by the same expert operator (GC) as follows:
- The operator moved 2 square marks (20 ´ 20 pixels), 1 red and 1 green, inside the lumen (blood reference) and over the brighter part of the artery wall (adventitia reference) (Figure 1A)
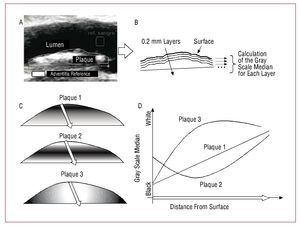
Figure 1. Bilayer method of echogenicity analysis. A: plaques were manually traced to define their contours. The 2 reference squares for the normalization of the intensity of the lumen and adventitia can be seen. B: plaques were divided into layers of 0.2 mm thickness, all parallel to the surface. The GSM was calculated for each layer and the depth curve produced. C: 3 plaques of different composition. D: Depth curves for each of the simulated plaques.
- Using the GSM of the pixels inside each square as a reference, the image was normalized so that all the pixels with a gray scale below that of the blood reference had a value of 0, and all those with a gray scale above that of the adventitia reference had a value of 255. The intermediate values were linearly adjusted.14,16 This normalization procedure compensates for the gain of the ultrasound scan and the structural differences inherent in each patient
- Via a number of clicks, the operator manually traced the boundary of the plaque, creating a polygon that enclosed it (Figure 1A). The number of pixels within this polygon was used to estimate the size of the plaque in that echographic projection. The size of each pixel was estimated using the calibration cursors of the ultrasound scan (between 102 and 140 pixels/cm for the normal level of magnification)
The analysis algorithm was then used to quantify the plaque automatically as described below:
- With respect to the position of the plaque (for or near wall), the pixels of the wall-lumen interface were identified and grouped to conform a surface curve (Figure 1B)
- For each pixel within the polygon outlined by the operator, the minimum distance to the surface curve was calculated
- After establishing layers, each 0.2 mm thick, the pixels were grouped according to their distance from the surface curve, stratifying the plaque into layers parallel to the wall-lumen interface (Figure 1B)
- For each 0.2 mm-thick layer, the GSM for all the pixels was calculated
Thus, a GSM curve was made for each plaque as a function of depth in 0.2 mm layers. The thickness of each layer was adopted as a function of the resolution of the ultrasound scan, estimated approximately as 0.1 mm. Both the traditional analysis and the bilayer analysis involved the surface 2 mm (first 10 layers); see Figures 1C and D for an example. Despite the fact that, globally, the gray scales of the 3 plaques shown are similar, only when the deep curves are studied can the plaque with a linear progression of density (plaque 1) be distinguished from that showing more opaque nuclei (plaque 2) or more dense nuclei (plaque 3). The algorithm requires a moderate computation time, but always under 5 s per plaque.
Statistical Analysis of the GSM Curves by Risk Group
Each plaque forms an independent unit of analysis associated with the clinical information of each patient. The values obtained are generally expressed as means SD.
The carotid and femoral plaques of the present patients were studied separately. Three risk factors were selected: age (using the median age as the cutoff point), sex, and smoking. Three comparisons were made, separating the plaques into 2 groups depending on the three risk factors outlined. To determine how these factors influenced the echogenicity of the plaques, both traditional and bilayer analyses were performed.
In the traditional method, the GSM was compared using the Student t test for non-paired data. Significance was set at P<.05. In the bilayer method, the GSM was averaged layer by layer in each group, thus making 2 mean curves. The comparison of the curves between groups was undertaken using 2-way ANOVA. The first factor was the "group", the second "depth." Therefore, a significant ANOVA results corresponds to 2 curves with different GSM in at least 1 layer. When such cases were detected, a post hoc Tukey honestly significant differences (HSD) test was performed and the dissimilar layers identified by marking the curves.
Interobserver Variation
A second operator analyzed a randomly selected subgroup of 55 plaques to examine the variation between observers with respect to the following variables: area, the GSM of the surface layer, and the median value for the first and second millimeter (known as 0, 1 and 2, respectively). The distribution of the area of the plaque was normalized using the logarithm function. Means, SD, and coefficients of variation (CV) [SD/mean)´100%] were recorded. Correlations were also calculated using the least mean squares methods. Trends were analyzed in the residuals graph.24
RESULTS
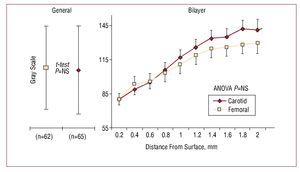
The risk of coronary heart disease in the study population, according to the Framingham scale, was 12% (8%) at 10 years. Table 1 shows the remaining clinical characteristics of the patients. A total of 127 plaques were found in the 42 patients. The echogenicity of the femoral and carotid plaques were similar in the traditional and bilayer analyses (Figure 2). As shown in the GSM curve, the echogenicity of the set of plaques tended to increase linearly towards their interior. The linear trend was significant (r=0.96; P<.001).
Figure 2.Bilayer analysis method of carotid and femoral plaques. Left: mean of the GSM and SD for the traditional method. Site had no influence on the gray scale; the Student t test returned a non-significant result. Right: reconstruction of the mean depth curves for both groups. The mean and standard error per layer is provided. Note that the curves are similar with a positive linear trend towards the interior of the plaque. Two-way ANOVA analysis returned a non-significant result.
Table 2 shows the results of the traditional analysis method. With respect to the femoral plaques, those of older patients were marginally more echogenic (P=.05). Neither sex nor smoking were found to significantly affect echogenicity. For the carotid plaques, neither age, sex nor smoking appeared to have any significant influence on echogenicity.
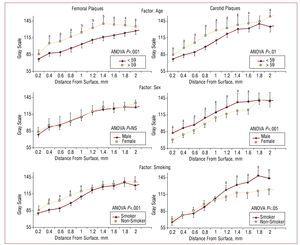
Figure 3 shows the results obtained using the bilayer method. The first line of information shows that the plaques of older patients to be more echogenic than those of younger patients, both for femoral plaques (ANOVA, P<.001) and carotid plaques (ANOVA, P<.01). In both cases, at least 9 out of 10 layers showed significant differences.
Figure 3.Reconstruction of GSM as a function of layer depth. Left: femoral plaques; right, carotid plaques. Each line of information separates 2 groups for 3 risk factors: age, sex, and use of tobacco. The curves were compared by 2-way ANOVA; the layers were then compared using the post hoc Tukey HSD test. aP<.05. bP<.01.
The second line of information shows that the femoral plaques of women and men subjects were of similar echogenicity, while more of the men's carotid plaques showed more noticeable echolucency(ANOVA, P<.01).
The third line of information shows that the surface regions of the femoral plaques of the smokers were less echogenic than those of non-smokers (ANOVA, P<.01). The nucleus of the carotid plaques of the smokers was brighter than that of the non-smokers (ANOVA, P<.05).
Interobserver Reproducibility
The SD and CV of the repeated measurements of the GSM made using the traditional method by 2 independent observers were 6.7% and 7.5%, respectively. Both measurements were strongly correlated (r=0.99; P<.001). For the 0, 1, and 2 mm layers, the SD and CV were 8.8% and 12%, 8.4% and 9.2%, and 8.9% and 7%, respecti vely. The correlation between observers for the 0, 1, and 2 mm layers were r=0.81 (P<.001), r=0.93 (P<.001), and r=0.99 (P<.001) respectively. The SD and CV for the log of the area of the plaques was 0.087 log mm2 and 6.6%, respectively. A strong correlation was also seen (r=0.97; P<.001). For all correlations the perceivable absence of trends in the analysis of residuals was verified.
DISCUSSION
This work extends the traditional analysis of the echogenicity of atheromatous plaques (which involves the calculation of the GSM of the pixels of lesions in general) by studying the layers running parallel to the plaque surface. While the variation between observers was similar in both methods, bilayer analysis was more effective at identifying the influence of risk factors on the echogenicity of the plaques. Age, sex, and smoking were all found to modify the structure of the plaques towards their interior—modifications only appreciable using the proposed method. The new method's greater specificity of stratification at depth compared to the traditional method is the major finding of the present study.
The vulnerability of an atheromatous plaque depends on its composition. In general, the most explored arterial segment is the carotid, although studies on the femoral artery show that the composition of plaques in this region can be used to efficiently predict cardiovascular risk.25,26 For this reason both types of artery were included in the present study. Lipid plaques, those with a lipid nucleus and a fine calcified layer at the surface, or those subject to internal hemorrhaging, are more likely to rupture, and are therefore termed vulnerable.16,22,23 A traditional analysis of the GSM in such cases is insufficiently specific to be able to detect such regional peculiarities. The new method, however, offers the equivalent of a virtual, layer-bilayer histological examination, in which the echogenicity of each layer is assessed. As shown in Figure 2, if, as a function of depth, the GSM curve is projected over the vertical axis, the information provided by the traditional analysis is reduced, with important information on the interior of the plaque remaining hidden. Figures 1C and D show plaques which, in general, have similar GSM but which can be clearly differentiated via their depth curves.
The proposed bilayer analysis increases the amount of information available on the composition of a plaque via its gray scales, but the technique requires a statistical analysis that compares 2 curves instead of 2 mean values. In this work, 2-way ANOVA was used with "group" and "depth" as the independent factors. To determine whether different risk factors influenced the composition of plaques, these were systematically divided into 2 groups, averaging their curves layer by layer. As shown in Figure 3, the new method allowed differences between the curves at different depths to be differentiated; these differences were not detected by the traditional method. The statistical results confirmed those observed visually. When the curves at different depths tended to entwine, the ANOVA result was not significant (Figure 2). However, when the layers tended to differentiate, post hoc analysis was sufficiently robust to pinpoint the exact location of the differences (Figure 3).
Figure 2 provides direct evidence of a further two findings: the linear trend in density as a function of depth, and the similarity in the echogenicity between segments. With respect to the former, the present data confirm the curves at depth reported by other researchers.20 The invasion of the lesion towards the lumen should be understood to represent an advanced stage of disease since the elastic remodeling phase has been completed. It is not contradictory that, as the analysis of echogenicity proceeds towards the adventitia, the tissue should become more dense. In fact, the adventitia is used as a reference for the highest gray scale.
The similarity in echogenicity of femoral and carotid plaques is confirmed by the curves shown in Figure 2. The normalization method may have been a factor that influenced this finding.27 In addition, the averaging of a large number of curves can produce a trend towards the mean. In order to identify vulnerable curve patterns, the analysis of the composition of the plaques of each patient becomes necessary. This would allow normality curves to be established, and therefore the detection of anomalies that might predict future ruptures.
The first line of information in Figure 3 shows that age is associated with more echogenic plaques in both sites. This is coherent with the natural history of atherosclerosis, with plaques becoming more fibrous over time.28 With respect to the factors sex and smoking, no significant differences were seen with regard to age. Thus, the differences seen in echogenicity must be explained by other factors. No differences were seen between men and women in terms of the echogenicity of femoral plaques, but the carotid plaques of the male patients were more echogenic than those of their female counterparts. This partially agrees with the results of a study involving 600 subjects in which echogenic carotid plaques were more commonly detected in men.6
Since men tend to develop plaques earlier7 their lesions may be older than those detected in women of the same age, and perhaps for this reason appear more dense. Interestingly, in another study with a similar number of patients, it was found that women had the more echogenic plaques.7 However, in this case the groups were not adjusted for age, and other factors such as the size of the plaques or cholesterol levels may have impeded adequate comparisons being made.
Finally, it was found that the femoral plaques of smokers were less echogenic at their surfaces than those of non-smokers, while the interiors of their carotid plaques were more echogenic than those of non-smokers. With respect to carotid plaques, this agrees with the results reported by other authors.6 The lower surface density of femoral plaques in smokers may explain the greater rupture risk associated with the use of tobacco.25 The discussion surrounding the influence of risk factors on plaque composition should be extended via future studies with more subjects to allow for the adjustments required.
The present work suffers from a number of limitations. The reproducibility of the quantitative methods for characterizing plaques has been discussed elsewhere.13-15 However, attempts to minimize variation included the involvement of a single expert observer who always used the same equipment for capturing, normalizing and processing the images.14,27 In the analysis of inter-observer variation, the CV was around 10% both for the traditional and bilayer methods for the surface, 1 mm and 2 mm layers. This similarity in variation in both methods appears reasonable since the technique is essentially the same, the difference lying in the automatic processing of the layer images. Repeatability was not analyzed since the follow-up of the patients did not so allow. It is important to note that only the first 10 layers of each plaque were subjected to echogenicity analysis. It was decided to study the region closest to the wall-fluid interface for 2 main reasons. First, the rupture of plaques has been associated with high shear stressesin the surface regions which, in the case of plaques covered with a fine fibrous layer, causes them to break and allow contact between the lipid content of the plaque and the blood. Second, since plaques vary in size, it was necessary to adopt a number of layers that would include the greatest number of lesions. The choice of the radial projections reflected the fact that the wall-fluid interface is the only interface that can be used as a reference for making parallel projections. No clear reference, either at the end or beginning of the lesions, was available for longitudinal projections. Studying the size of the lesions was not, however, a major objective of the present work. Future protocols should be designed to focus on the area of plaques and its relationship with lesion composition. Other authors who have advanced in the stratification of plaques have opted to quantify plaque composition via gray thresholds and by giving color to the different regions.20 The statistical method outlined in the present work for analyzing the differences between curves might help perfect this approach. Finally, work still needs to be performed in symptomatic patients or patients receiving secondary attention in order to progress in the obtaining of reference curves that would allow the vulnerability of atheromatous plaques to be examined early. This would help in decisions regarding individual preventive treatment.
CONCLUSIONS
Image processing techniques make it possible to stratify the echogenicity of atheromatous plaques by layers, and to construct GSM curves as a function of layer depth. Grouping plaques by patient risk factors means these curves can be used to study plaque echogenicity in much greater detail than with the traditional analysis method. Being able to quantitatively describe regional configurations of lesion structure as a function of depth is the first step towards making an advance in our estimation of plaque vulnerability to rupture.
ABBREVIATIONS
CV: coefficient of variation
GSM: gray scale median
SD: standard deviation
This work was partially funded by the ECOS-SUD program, project No. A06S02 (Argentina-France).
Correspondence: Dr. D. Craiem.
Avda. Belgrano 1723. (1093) Ciudad de Buenos Aires. Argentina.
E-mail: dcraiem@favaloro.edu.ar; damian@craiem.com.ar
Received October 14, 2008.
Accepted for publication June 10, 2009.