Keywords
INTRODUCTION
Early clinical studies demonstrated that oral anticoagulants based on vitamin K inhibition were better than antiplatelet agents for reducing vascular events in patients with atrial fibrillation (AF).1 More recently, other studies—designed as non-inferiority studies—have compared antithrombotic drugs and vitamin K antagonists, indicating that the former were not superior.2 In fact, the National Study for Prevention of Embolism in Atrial Fibrillation (NASPEAF, Estudio Nacional para la Prevención del Embolismo en la Fibrilación Auricular) was designed as a "superiority study" and demonstrated that combined antithrombotic treatment (anticoagulation treatment at therapeutic doses plus triflusal 600 mg/d) was more efficient than anticoagulation monotherapy (INR, 2.0-3.0), with a lower incidence of hemorrhagic complications.3-5 The combination therapy arm in the high-risk patient group had a median INR of 2.17, and the P25-P75 interquartile range was 1.97-2.36. Given these results, the patients receiving combination therapy with a target INR ranging from 1.9 to 2.5 underwent further follow-up. Those receiving standard anticoagulation therapy were assigned to the control arm. To investigate a possible alternative antiplatelet strategy for combined therapy, 2 groups of patients were created who received similar levels of anticoagulants plus either triflusal 300 mg/d or acetylsalicylic acid (ASA) 100 mg/d.
METHODS
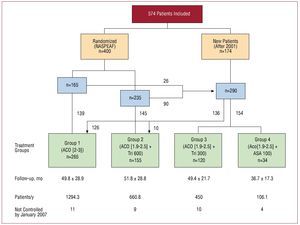
The study included 574 patients with AF who attended the outpatient cardiology unit at the Hospital Clínico San Carlos in Madrid (Spain), and associated medical centers. They were consecutively enrolled during the previous 11 years for prospective follow-up (Figure 1). A total of 400 patients in the NASPEAF study had been randomly assigned to either standard anticoagulation treatment or combined anticoagulant therapy plus triflusal at 600 mg/d. Of the latter, 264 continued with the randomized treatment, 20 withdrew from it and the remaining 116, together with another 174 new patients, received anticoagulation monotherapy or combined therapy with different antiplatelet agents. Changes in the anticoagulant or antiaggregation treatment was left to the discretion of the attending doctor and the preferences of the patients. Most patients receiving combination therapy with ASA presented coronary heart disease. The patients receiving combination therapy with triflusal 300 mg/d received anticoagulant at therapeutic doses (INR >2.0), and some antiplatelet effect was achieved by the addition of triflusal 300 mg/d.
Figure 1. Flow chart of randomized and new patients. ACO, anticoagulant; ASA, acetylsalicylic acid; INR, international normalized ratio; Tri, triflusal.
The final groups were as follows: group 1 (n=265) received anticoagulation monotherapy for a target INR in the range of 2.0-3.0; groups 2-4 received combined therapy with anticoagulants for a target INR of 1.9-2.5 plus triflusal 600 mg/d (group 2 =155 patients), triflusal 300 mg/d (group 3 =120 patients), or ASA 100 mg/d (group 4 =34 patients). The mean follow-up times in each group were 49.8 months, 51.8 months, 49.4 months, and 36.7 months, respectively, with corresponding values of 1294.3 patient/y, 660.8 patient/y, 450 patient/y, and 106.1 patient/y.
At the time of inclusion, the prevalence of the main risk factors (previous embolism and >75 years of age) were recorded together with the following quantitative factors: weight, height, heart rate, and left ventricular and atrial diameters measured by M-mode echocardiography (Table 1). The prevalence of the following qualitative risk factors were also recorded: hypertension, sex, a history of heart failure, diabetes mellitus, dyslipidemia, cardiomyopathy, coronary heart disease, and a history of smoking. The prevalence of the risk factors was analyzed and compared in the different groups of patients as well as their predictive value for vascular events or severe bleeding.
The analysis only included those patients who had undergone a minimum follow-up of 12 months. The hospital records of the patients who had not undergone control in 2006 were checked, and telephone contact attempted if no data were available. Nearly all patients had been contacted by January 2007, except for 5.9%.
International normalized ratio control was conducted regularly in 2 anticoagulant units (total number of controls, 11 358). The INR value was also recorded when hospital admission took place due to a vascular or hemorrhagic event. The mean INR for the total follow-up period was analyzed as well as the percentage of INR samples within the therapeutic range, below the lower range, and above 3.5. Gastroscopy was conducted in all patients who required hospital admission due to suspected gastric bleeding.
The following were considered as primary endpoints: ischemic or hemorrhagic stroke, systemic embolism, acute coronary syndrome, sudden death, and death within 30 days of an event or severe bleeding.
The definitions of these endpoints were described in previous publications.3-5 A diagnosis of coronary syndrome was established if there was characteristic retrosternal pain associated with electrocardiographic changes or elevated levels of cardiac enzymes.
The primary endpoints and severe bleeding were analysed and compared between the different groups of patients. These events were also compared between the NASPEAF study period (1995-2001) and after June 2001 in the randomized groups of patients (groups 1 and 2). The patients in these groups were 3.3 years older at the beginning of the second period.
From 2003 onwards, omeprazole 20 mg/d was administered gradually to patients assigned to the combination therapies. The patients receiving the latter therapy formed 14.6%, 40%, and 40% of groups 2, 3, and 4, respectively.
Statistical Analysis
Discrete variables were compared at baseline using Fisher's exact test or the c2 test and were expressed as percentages; continuous variables were assessed using ANOVA and expressed as mean values and standard deviation. Baseline comparisons were adjusted by multivariate analysis. The incidence of events was expressed as events per 100 patient/y. The Cox proportional hazards model was used to identify the risk factors for vascular events and severe bleeding, and the variables (previous embolism, >75 years of age, and coronary heart disease) were adjusted for the different therapeutic arms. The Kaplan-Meier method was used to calculate the event-free survival curves up to the first event. Risk estimation was adjusted and satisfied comparing the estimated ln (-ln) of the survival curves transformed into parallel curves. Finally, the hazard ratio (HR) and 95% confidence interval (CI) of the Cox regression model were calculated. All the tests were conducted using a P-value of <.05 as a cutoff for statistical significance. All statistical analyses were conducted using SPSS version 15.0 for Windows.
RESULTS
The incidence of risk factors was similar in the first 3 groups, except for a greater prevalence of previous embolism in group 2 and dyslipidemia in group 3 (Table 1). The group receiving ASA (group 4) had a higher prevalence of previous embolism, smoking, male sex, ischemic heart disease and older age, although the prevalence of previous embolism had values similar to group 2.
The Cox proportional hazards model identified the following risk factors as independent predictors of vascular events: history of previous embolism (HR=3.52; P<.001) and coronary heart disease (HR=3.01; P=.001). Being older than 75 years showed a slight, but nonsignificant, statistical trend (HR=1.51; P=.186). Previous embolism and coronary heart disease were also predictors of severe bleeding, and reached statistical significance: HR=2.40 (P=.004) and HR=2.75 (P=0.002), respectively. Other variables (dyslipidemia, male sex, and smoking) were also heterogeneously distributed between the comparison groups, but were not included in the final model because of their high correlation with the variable coronary heart disease.
Mean INR values and other anticoagulation parameters in group 1 were significantly higher than those found in the combined therapy groups (2-4), but were very similar within groups 2-4 (Table 2). The mean INR values at hospital admission due to ischemic events in groups 1, 2 and 3 were 2.09, 1.92 and 1.57, respectively (Table 2). The INR values were below 1.9 in all patients in group 3 who had been admitted at hospital for ischemic events, and no event of this kind was recorded in the ASA group. Mean INR values in patients from groups 1-4 admitted to hospital for severe bleeding were 4.32, 2.77, 4.24, and 5.5, respectively.
Event rates (and mortality rates) in groups 1-4 were 2.86 (1.24), 1.36 (0.91), 2.67 (0.89), and 2.83 (2.83), respectively (Table 3). The rates of ischemic events were 1.70, 0.61, 2.44, and 0, respectively. The patients in the combined therapy group receiving triflusal 600 mg/d presented significantly fewer primary endpoints than those who received anticoagulation therapy alone (1.36 vs 2.86; P=.039). When only the randomized patients in these 2 groups were taken into account, these rates were 1.48 vs 3.37, with a similar difference (P=.04) (Figure 2). Event rates during the NASPEAF study period (1995-2001) and after June 2001, were 3.53 and 2.38 in the anticoagulation monotherapy group, and 0.97 and 2.76 in the combined therapy group (Table 3). Neither comparison reached statistical significance. In the combined therapy group, the ischemic event rate was similar in both periods; the difference in the number of overall events was due to an incidence of sudden death which was 3 times higher in the second period. Event rates, without adjusting for predictors, were similar in groups 1, 3, and 4. The groups receiving combined therapy with triflusal 300 mg/d or ASA 100 mg/d had a nonsignificant trend of a greater number of events than the group receiving combined therapy with triflusal at 600 mg/d.
Figure 2. Survival curves for combination therapy (anticoagulant + triflusal 600 mg/d) versus anticoagulants alone. A: all patients. B: randomized patients.
When the predictor variables were adjusted using the Cox regression model, the significant benefit of the combination therapy in group 2 was confirmed when compared to anticoagulation monotherapy (HR=0.33 [0.14-0.80]; P=.014). Significant benefit was also found in the group receiving triflusal 300 mg/d (HR=3.07 [1.11-8.50]; P=.031) (Figure 3). Patients who received combined therapy with triflusal 300 mg/d and those who received anticoagulation therapy alone presented the same event rate (HR=1.02; P=.957), but the latter group had a higher rate of severe bleeding than the former.
Figure 3. Hazard ratio (HR) after adjusting for risk factors in the different therapeutic groups. A: combination therapy group 2 (anticoagulant + triflusal 600 mg/d), group 3 (anticoagulant + triflusal 300 mg/d), and group 4 (anticoagulant + ASA 100 mg/d) versus group 1 (anticoagulant only). B: combination therapy group 3 and group 4 versus group 2. ACO, anticoagulants; ASA, acetylsalicylic acid; Tri, triflusal. *No ischemic events were reported in the combination therapy plus ASA group.
The total rate of severe bleeding in groups 1, 2, and 3 were 2.47, 1.51, and 1.33, respectively, without significant differences between comparisons, although mortality was greater in group 1 (Table 3); of the 32 cases of severe bleeding recorded in this group, 5 were intracranial and 4 patients finally died. Another 6 patients presented gastric bleeding (2 patients died and the rest required transfusion). The rate of non-gastric bleeding in the anticoagulant group (2.01) was significantly higher than in the groups receiving combined therapy with triflusal 600 mg/d or 300 mg/d (event rates, 0.30 and 0.22; P=.012 and P=.004, respectively). The event rate recorded in the ASA group (6.60) was significantly higher than in the other groups (P=.008); all cases involved non-gastric bleeding.
Most bleeding events in the combined therapy groups receiving triflusal involved gastric bleeding, and endoscopic studies showed superficial stomach disease involving the mucous membrane. The incidence of gastric bleeding in these groups decreased progressively from 2002 onwards as the patients received proton pump inhibitors.
DISCUSSION
Primary Endpoints in Studies on Stroke Prevention in Atrial Fibrillation
Classic articles on stroke prevention in atrial fibrillation based their conclusions on the incidence of ischemic events.6-9 In our opinion, and in line with the CONSORT Statement recommendations,10 serious complications from bleeding events, especially death from bleeding, should be taken into account as primary endpoints. In fact, the SPAF II study drew different conclusions after considering intracranial bleeding and vascular death as primary endpoints. On the other hand, a critical analysis of classic studies that included vascular death as primary endpoint led Oden et al11 to suggest reducing the anticoagulation level to a target INR in the range 2.0 to 2.5.
Anticoagulation Intensity in Combined Antithrombotic Therapy
In the NASPEAF study, the mean INR value during combination therapy was 1.97 in the group at medium risk and 2.17 in the high-risk group, with a P25-P75 semiquartile range of 1.97-2.36. The difference was small and there were numerous values which overlapped between the 2 groups. Thus, we could not justify establishing 2 different therapeutic regimens in any future study, and proposed changing the previous target range of 1.4-2.4 to 1.9-2.5. The lower limit of the INR range was set at 1.9-2.0 in the patients receiving anticoagulant monotherapy.12,13 An anticoagulation level below 2.0 in combination therapy could be suggested, but it is unclear how low this level could be set14: very low values (mean, 1.3) failed to prevent ischemic vascular events in some early studies,15,16 and coagulation control could not be avoided in any of the patients. Our current results (Table 3, Figure 3) confirm that combination therapy with a target INR between 1.9 and 2.5 is significantly more efficient than anticoagulant monotherapy, and has a very low rate of non-gastric bleeding.
Platelet Activity and the New Coagulation Cascade: The Role of Acetylsalicylic Acid
The new coagulation cascade proposed by Monroe et al17 in North Carolina, and Shafer18 in Houston, is the result of painstaking research conducted after the classic cascade was described by MacFarlane19 in 1964. The new cascade, accepted by the Working Group of the European Society of Cardiology,20 might help to explain the benefits of antithrombotic combination therapy versus anticoagulant monotherapy. According to the description of the new cascade, platelet activity plays a key role in the activation of coagulation factors during cascade amplification and self-propagation phases. Consequently, platelet activity inhibition, in the presence of therapeutic concentrations of prothrombin inhibitors, can facilitate a greater decrease in thrombin generation. Anticoagulation monotherapy is effective in controlling vascular events in patients with AF at moderate risk, but it does not offer the same benefit to patients at high risk. Thus, in the EAFT12 study, the event rate in patients with a previous history of embolism was 8.5, whereas the event rate was 11.1 in our group of patients with a previous history of embolism and who were older than 75 years.5 On the other hand, the studies on factor X or thrombin inhibitors have not demonstrated superiority over vitamin K inhibitors 20 in solving this problem. More studies are needed that are aimed at improving the benefit of anticoagulation monotherapy in patients at high risk; these patients represent between 28% (patients with previous embolism) and 25% (>75 years of age) of the total number of patients with AF.3
Combined antithrombotic treatment became discredited after the SPAF III study,5 but the level of anticoagulation in this study was below therapeutic dosage and the ASA dose was too large. The NASPEAF study demonstrated that combined therapy with triflusal 600 mg/d offers a significant benefit for preventing vascular events compared to standard anticoagulant treatment.3-5 The long-term follow-up conducted over 11 years in randomized patients confirms our previous results, which described a very low incidence of non-gastric bleeding. At present, any potential benefit that could be added to standard anticoagulation has to be achieved by the addition of some platelet activity inhibitor. Our group has successfully used triflusal, which is a weaker cyclooxygenase inhibitor than ASA. It may be the case that platelet activity inhibition does not have to be very powerful to ensure a sufficiently strong antithrombotic effect in combined therapy with anticoagulants. No superior benefit was demonstrated with triflusal 300 mg/d as an alternative antiplatelet strategy, whereas ASA 100 mg/d produced a very high incidence of severe hemorrhagic events. The anticoagulation level was the same in the 3 groups of patients who received combined therapy, but the treatment was not randomized; some groups had few patients, and the prevalence of risk factors differed, although this was taken into account when the adjusted Cox regression model was applied to the predictor variables. Therefore, these results should not be used to reject any role for ASA in combined therapy, but they do demonstrate that the best dose remains unknown: >300 mg/d, used in earlier studies21 was associated with a risk high of bleeding, as occurred with the 100 mg/d dose used in our study. On the other hand, a recent randomized study conducted with patients presenting coronary heart disease,22 demonstrated that 75 mg/d combined with intense anticoagulation (INR, 2.0-2.5) similar to the one used in our group, did not significantly increase the rate of bleeding complications. Therefore, future studies are needed to evaluate the role of ASA in antithrombotic combined therapy
Gastric Bleeding During Combined Antithrombotic Therapy
Most gastric bleeding was recorded in patients assigned to combined therapy with triflusal who did not receive proton pump inhibitors. Once the NASPEAF study analysis was completed, and from 2002 onwards, proton pump inhibitors were administered to patients receiving combined therapy in the present study. In group 2, a total of 7 gastric bleedings were recorded before June 2001, and only 1 after the gradual introduction of gastric protection agents. In group 3, some patients presented gastric bleeding events, but these only occurred in those who did not receive omeprazole. During the administration of combined therapy, gastric endoscopy—which was conducted in all patients admitted to hospital for gastric bleeding—indicated superficial damage to the mucous membrane, which has been associated with the acid component of the antiplatelet drug.23,24 Less damage is caused when the drug is administered with an enteric coating,23 and can be prevented by adding proton pump inhibitors to the treatment.25 None of the patients included in the ASA group presented gastric bleeding, probably due to the low acid component of ASA 100 mg and the use of tablets with an enteric coating.
The part of the study aimed at investigating alternative antiplatelet treatment strategies had some limitations: a) the low number of patients, especially in the ASA group; b) although data collection was prospective, allocation to treatment was not randomized; c) the prevalence of risk factors was not homogeneous, although they were adjusted for the Cox regression model; and d) limitations in the interpretation of results characteristic of studies using combined endpoints.
CONCLUSIONS
Outstanding questions concerning the benefit of ASA in combination therapy justify a randomized multicenter study using low doses of the drug.
ABBREVIATIONS
AF: atrial fibrillation
ASA: acetylsalicylic acid
NASPEAF: Estudio Nacional para la Prevención del Embolismo en la Fibrilación atrial (National Study for Prevention of Embolism in Atrial Fibrillation)
SEE EDITORIAL ON PAGES 972-5
Correspondence: Dr. R. Bover Freire.
Secretaría de Cardiología. Hospital Clínico Universitario San Carlos. 28040 Madrid. España.
E-mail: ramonbover@secardiologia.es
Received July 16, 2008.
Accepted for publication April 14, 2009.