Keywords
INTRODUCTION
Patients who arrive at hospital with prolonged chest pain, elevated levels of cardiac enzymes, and electrocardiographic changes (ECG) are admitted with an initial diagnosis of acute coronary syndrome (ACS). Of these patients, between 7% and 10% do not present significant coronary lesions and it is often difficult to establish a precise diagnosis.1 Sometimes the situation is one of acute myocardial infarction without angiographic features of coronary lesions at the time of the study, but other nonischemic disorders exist that can present a clinical picture similar to ACS, such as myocarditis or transient apical dyskinesia.2,3 Establishing a correct diagnosis has important prognostic and therapeutic implications for the patient.
Cardiac magnetic resonance (CMR)—especially when used with gadolinium delayed contrast-enhancement—is a highly sensitive non-invasive imaging technique for detecting myocardial abnormalities. One of its most well-known uses is in the detection of areas of myocardial infarction, but it is also possible to visualize fibrotic or necrotic areas in other diseases of non-ischemic origin.4,5 Given that prognosis and therapeutic management will radically differ depending on whether we are dealing with an acute coronary episode or a nonischemic clinical picture, reaching a precise diagnosis is of great importance in these patients.
Our aim was to establish whether CMR study in patients admitted with a clinical picture of ACS with normal coronary arteries is of help in the diagnosis of these patients.
METHODS
Between January 2004 and December 2007, we studied 80 patients admitted to hospital for prolonged chest pain at rest (>30 min), with elevated levels of markers of myocardial damage. They were referred to the cardiac catheterization laboratory with an initial diagnosis of ACS, and all had findings of some coronary arteries without significant lesions. These patients underwent 8% of all catheterization procedures conducted in patients with suspected ACS. Patients with anterior myocardial infarction were excluded from the study as well as those patients with some other disorder involving elevated troponin levels (1 patient with chronic degenerative muscle disease and 1 with advanced chronic kidney failure) and patients in whom a magnetic resonance study was contraindicated (2 patients with pacemakers and 1 patient with claustrophobia).
All patients underwent serial electrocardiogram, serial measurement of myocardial damage marker levels, chest x-ray, echocardiogram, coronary angiography, and CMR study. Cardiac catheterization and echocardiogram were performed in all patients no later than 48 h after admission. All CMR studies were conducted 4 (3) days after cardiac catheterization.
Cardiac Magnetic Resonance Study
All CMR studies were conducted at 1.5 T field strength (Siemens Symphony® and Philips Intera®) with a multi-element phased-array coil optimized for cardiac studies. The following steps were performed:
1. Cine mode (steady-state free-precession [SSFP]) with 24 or more cardiac phases/cycles, and slice thickness 8 mm + 2 mm gap or 10 mm without gap. The recorded views were as follows: 2-chamber, 4-chamber, 3-chamber, short-axis with complete coverage of the left ventricle (LV) from the mitral valve view to the apex.
2. T2 black-blood weighted imaging (TSE-STIR or triple inversion; alternative TSE-SSFP) in 3 short-axis slices (basal, midventricular, and apical) and long-axes (2c, 4c, 3c) slices. Slice thickness, 10-15 mm; TR =2 RR intervals; TE, 65 ms; field of vision, 320-380 mm; 256´256 matrix.
3. Delayed-enhancement imaging: T1 weighted inversion-recovery gradient-echo imaging (Turbo Flash on the Siemens instrument and T1-Turbo Field Echo on the Philips instrument) performed 10 min after the administration of 0.2 mmol/kg gadolinium-DPTA in the same view as the images in cine mode. Inversion time was individually adjusted to null the signal from healthy myocardium, and this varied between 225 ms and 275 ms. The field of vision ranged between 320 mm and 380 mm and the matrix was 256´256.
4. In the last 37 patients in the series, a perfusion study at rest was also performed during the administration of 0.5 mmol/kg gadolinium-DPTA.
The CMR studies were assessed by 2 expert interpreters. Ventricular volumes and left ventricular ejection fraction (LVEF) were measured. Left ventricular wall motion was measured according to the classification of the American Heart Association.6 The T2 weighted images (TSE-STIR) were studied to detect potential areas of high signal-intensity compatible with myocardial edema. The T2 weighted images were measured by visual estimation without using any quantification method. Finally, these images were analyzed to detect areas of subendocardial, intramyocardial, subepicardial, and transmural contrast enhancement. The final diagnosis was based on the delayed contrast-enhancement images. The patients who presented areas of subendocardial and transmural contrast enhancement had a diagnosis of myocardial infarction. The patients with subepicardial and intramyocardial contrast enhancement had a diagnosis of myocarditis.
RESULTS
Clinical and Echocardiographic Findings
The results are presented Table 1. Most of the patients were men with a mean age of <50 years. High levels of troponin T were observed in all the patients and high levels of creatine kinase in 69 patients. The most frequent electrocardiographic changes were ST-segment elevation (40%) and ST-segment depression (26%). In the echocardiographic study, 51 (63%) patients presented wall motion abnormalities which occurred with greater frequency in the lateral or lower segments of the LV (44 patients); 10 patients presented severe contractile abnormalities with dyskinesia in all apical segments; 14 patients in the series only presented increased ventricular volumes and 26 had echocardiographic LV dysfunction at admission. Mild pericardial effusion was detected in 13 patients. There were no echocardiographic findings of significant abnormalities in 21 patients.
Cardiac Magnetic Resonance Findings
The findings of the CMR study are presented in Table 2 and Table 3.
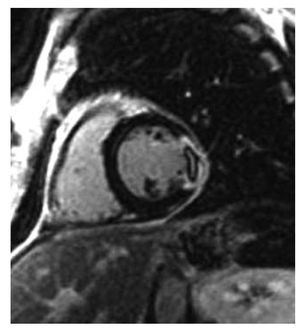
The ejection fraction was slightly higher than that measured by echocardiography. The results of the wall motion study were similar to those estimated by echocardiography, with the exception of 5 patients who presented extensive echocardiographic apical dyskinesia, although this had disappeared by the time of the CMR study. On the T2 weighted images, we detected a hyperintense signal compatible with edema in 42 patients; this was located in the septal or anterior segments in 10 patients and in the lateral and lower segments in the remaining patients. On the delayed contrast-enhancement images, we detected areas of subendocardial contrast enhancement—all of which were located in the lower segments—in 8 patients and areas of transmural contrast enhancement in 4 patients; 2 with an anterior location, 1 with a lateral location, and 1 with an inferior location. These 12 patients who presented subendocardial or transmural contrast enhancement had a diagnosis of myocardial infarction; images of perfusion defects typical of "microvascular obstruction" (Figure 1) were found in 2 of these patients; 4 of the patients who had a diagnosis of acute myocardial infarction (AMI) presented a hyperintense signal compatible with myocardial edema on the T2 weighted imaging study.
Figure 1. Inversion-recovery images in the short-axis view showing a transmural lateral myocardial infarction with perfusion defect (microvascular obstruction).
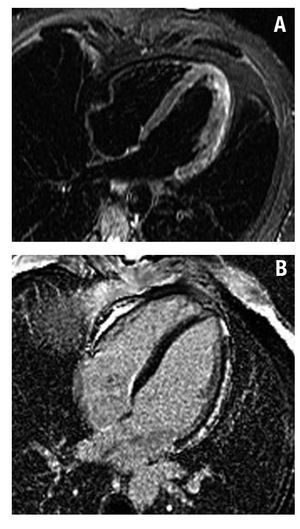
In 51 patients, we found areas of subepicardial or intramyocardial contrast enhancement mainly located in the LV lateral segments. The contrast enhancement pattern was multifocal and patchy in 39 patients; 35 patients presented a hyperintense signal on T2 weighted images; these 51 patients had a diagnosis of acute myocarditis (Figures 2 and 3).
Figure 2.A: STIR images in 4C view showing hyperintense signal in the lateral wall of the left ventricle (edema). B: inversion-recovery images showing subepicardial delayed contrast enhancement in the lateral wall of the left ventricle, typical of myocarditis.
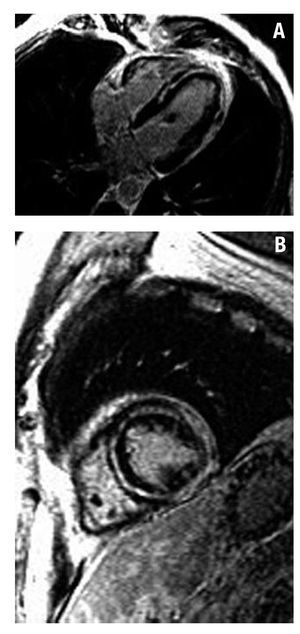
Figure 3. Inversion-recovery images. A: 4C view. Intramyocardial delayed contrast enhancement in the septum and apex and lateral subepicardial enhancement (myocarditis). B: short axis view of the left ventricle showing subepicardial contrast enhancement and extensive intramyocardial contrast enhancement.
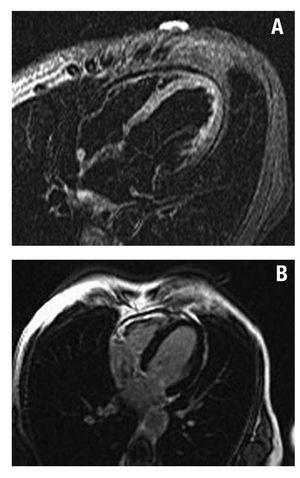
There were no findings of contrast enhancement on inversion-recovery images in 17 patients. Nine of these patients had a diagnosis of takotsubo cardiomyopathy after echocardiographic wall motion abnormalities were normalized and in the absence of contrast enhancement in the CMR study that ruled out AMI or myocarditis. All the patients except one were post-menopausal women with an average age of 64 (11) years; 7 patients reported previous emotional stress. The first echocardiogram showed severe contractile abnormalities in the apical and medioventricular segments. Basal LVEF was 36% (6%). After 7 days, LVEF was 62% (6%). There were findings of edema in 3 of these patients on T2 weighted imaging (Figure 4). Progress was good in all cases and there was 1 recurrence during an average follow-up of 13 months.
Figure 4. Patient with a diagnosis of takotsubo cardiomyopathy. A: STIR images showing a hyperintense signal in apical segments (edema). B: inversion-recovery images demonstrating the absence of delayed contrast enhancement.
Of the 8 remaining patients in whom contrast enhancement was not detected, 4 had a diagnosis of pericarditis, whereas no diagnosis could be established in 4 at hospital discharge.
DISCUSSION
Twelve-lead electrocardiogram and changes in levels of markers of myocardial damage are the basic tools in the diagnosis of patients with chest pain. However, it is well known that abnormalities in ECG and elevated troponin levels can lead to false-positive diagnoses. A total of 10% of the patients who have been initially diagnosed with ACS due to ECG abnormalities and elevated troponin levels have angiographically normal coronary arteries.1,2 In this context, CMR is an ideal method for characterizing myocardial abnormalities, especially when analyzing the presence and distribution of gadolinium contrast enhancement. In addition, there is abundant information in the literature on the contribution of T2 weighted imaging in the detection of myocardial edema, although its added value to delayed contrast-enhancement is a matter of debate. In a study by Assomull et al,3 the T2 weighted images were assessed by visual estimation without contributing relevant information to the final diagnosis of the patients, as we observed in our series of patients. There is a broad consensus in the literature that CMR with gadolinium enhancement is a powerful diagnostic method to detect necrosis and myocardial fibrosis both in ischemic heart disease and in other types of non-ischemic lesions.5 In our series, we demonstrate that a CMR study enables the etiological origin of the clinical picture to be identified in a high percentage of patients with an initial diagnosis of ACS and who have normal coronary arteries. The most frequent cause was myocarditis (63%), followed by acute myocardial infarction (15%) and takotsubo cardiomyopathy (11%).
Myocarditis Presenting as ACS
In our series, this was the most frequent confounder and is in line with the recently published study by Assomull et al,3 who reported a slightly lower incidence (50%) of a diagnosis of myocarditis in patients with an initial diagnosis of ACS and who had normal coronary arteries.
The explanation for the high frequency of diagnostic errors involving myocarditis is based on its diagnostic difficulty using standard methods. The clinical findings of myocarditis are often limited to minor signs such as dyspnea, chest pain, or palpitations on the days following a febrile episode, but the initial clinical symptoms often resemble acute myocardial infarction, with ECG abnormalities and elevated levels of markers of myocardial damage.7-9 Due to the limited specificity of its clinical symptoms of presentation, myocarditis is difficult to recognize clinically during its initial episode, and is therefore likely to be an underdiagnosed disease.
Endomyocardial biopsy is considered the reference technique for the diagnosis of myocarditis10; however, this technique has low sensitivity (<50%). Myocarditis frequently presents as a focal disorder and this fact plus sampling errors probably form the basis for its low sensitivity.11 In addition to these limitations, endomyocardial biopsy is a technique which is not free from complications, since several biventricular samples have to be collected, which involves left and right catheterization. Currently, it is only considered indicated if the patients have a poor clinical course and the information obtained can modify the treatment. In our study, no patient underwent endomyocardial biopsy. Cardiac magnetic resonance has become the imaging technique of choice for the diagnosis of myocarditis.12-17 Edema and inflammation lead to an increase in the content of cellular and extracellular water, which alters T2 relaxation times, identified as areas of increased signal intensity on T2 weighted images.
In our study we found areas of edema in 37 patients with myocarditis. In all of these patients, the CMR study was conducted within 7 days of the acute episode. As described in previous studies, edema disappears within 7 days of the acute episode, and thus the patients who are studied later do not present these abnormalities on T2 imaging.
Delayed contrast-enhancement imaging visualizes areas of uptake in relatively early phases, usually with a patchy and multifocal pattern, that extends from the subepicardium to the middle areas of the myocardium, except for the subendocardium which makes it possible to distinguish it from myocardial contrast enhancement in acute myocardial infarction which always affects the subendocardium.18,19 The fact that these areas of contrast enhancement correspond to foci of myocarditis was demonstrated by Mahrholdt et al18 in a landmark study in which all patients underwent CMR and endomyocardial biopsy, and in which the samples were taken from the areas where CMR showed contrast enhancement. Subsequently, the same authors described different types of contrast enhancement associated with the form of clinical presentation and the causative agent.19 Patients who are admitted with a clinical picture of chest pain and elevated levels of enzymes compatible with ACS have subepicardial enhancement that predominates in the LV lateral wall. All the patients in our series had a clinical presentation of chest pain and the most frequent location of enhancement was the LV lateral segments. Of the 51 patients who had a diagnosis of myocarditis, 34 presented wall motion abnormalities and only 11 presented left ventricular dysfunction. The reason for this is that we only included patients with myocarditis who had a clinical presentation of chest pain and elevated levels of enzymes, whereas patients with myocarditis which began as heart failure were excluded, and these are the cases that involve ventricular dysfunction. Another explanation found in the literature is that, due to the predominance of subepicardial involvement in this disease, the impact on contractility is much less than in the processes which involve subendocardial or transmural areas.
In view of the fact that all the patients evolved well after the symptoms had disappeared within the first days, and without recurrence or signs of heart failure, analgesic treatment only was administered and other studies were not considered appropriate.
AMI With Normal Coronary Arteries
The usefulness of CMR to study myocardial infarction has been widely demonstrated in multiple studies. The area of enhancement accurately identifies the area of myocardial necrosis and its transmural extent indicates the non-viable territory.20-24 In our series, 13 (15%) patients had a diagnosis of myocardial infarction, a figure comparable to that found by Assomull in a similar series, in which 11.6% of the patients with suspected ACS and with normal coronary arteries had a final diagnosis of AMI after CMR study. Of our patients diagnosed with AMI, infarction was transmural in 4 patients, 2 of whom had findings of "microvascular obstruction." In 3 of the patients studied 72 h after admission, we detected findings of edema on T2 weighted images. We did not find this abnormality in the other patients with infarction and who were studied later.
These patients did not undergo additional studies to investigate the possible mechanism of the transient coronary obstruction that could be associated with coronary spasm, embolism, or recanalization of epicardial arteries.
Takosubo Syndrome as the Final Diagnosis
In our series, the final diagnosis was takotsubo cardiomyopathy in 11% of patients. It was diagnosed on the basis of transient wall motion abnormalities and the absence of enhancement on the CMR study that ruled out AMI or myocarditis. In the CMR study, we detected increased STIR signal intensity at the level of the apical myocardial segments, compatible with edema, in 3 patients who underwent very early CMR study (<48 h). We did not detect delayed contrast enhancement in any patient on the images obtained after gadolinium administration, which ruled out infarction and myocarditis. These findings agree with those reported in the literature that describe the absence of contrast enhancement as characteristic of takotsubo cardiomyopathy on CMR study.25-27
Limitations
In a small percentage of cases (5%), it proved impossible to establish the diagnostic origin of the myocardial lesion demonstrated by the electrocardiographic changes and the elevated levels of biochemical markers. These patients had lower troponin T levels (1.5 [0.7]) and one of them presented obvious inferior wall motion abnormalities. We cannot rule out the possibility that the absence of contrast enhancement on the CMR study was due to the fact that the technique is limited by inadequate spatial resolution when detecting very small necrotic areas.
Delayed contrast enhancement and T2-STIR images were assessed by visual estimation alone. Our aim was to conduct a study that could be easily transferred to clinical practice; a T2-STIR and delayed contrast enhancement quantitative study would have hindered image acquisition (by preventing the use of a multi-element phased-array coil or that would have required the use of difficult to acquire adjustment algorithms) and image postprocessing. In fact, this approach may have limited the sensitivity of T2-STIR imaging to detect inflammation. However, we can conclude that the visual estimation of delayed contrast enhancement images allows a very high diagnostic yield to be obtained both simply and intuitively.
CONCLUSIONS
A number of non-ischemic diseases can present with symptoms similar to ACS. In view of the fact that a correct diagnosis has important prognostic and therapeutic implications for the patient, a CMR study and especially assessment of the presence, distribution and pattern of delayed contrast enhancement on CMR are of immense help in establishing a diagnosis.
ABBREVIATIONS
ACS: acute coronary syndrome
AMI: acute myocardial infarction
CMR: cardiac magnetic resonance
ECG: echocardiogram
SEE EDITORIAL ON PAGES 966-71
Correspondence: Dra. E. Laraudogoitia.
Servicio de Cardiología. Hospital de Galdakao.
Barrio Labeaga, s/n. 48960 Galdakao. Vizcaya. España.
E-mail: elaraudogoitia@hotmail.es
Received January 4, 2009.
Accepted for publication March 27, 2009.