Pregnancy in women with Marfan syndrome (MS) is associated with an increased risk of aortic events. The clinical evidence on pregnant patients with MS is limited and there is no specific consensus on their optimal management. We report our multicenter experience.
MethodsFrom January 2004 to January 2020, 632 patients with MS underwent periodic monitoring in Marfan units. During this period, we identified all pregnant women with MS and analyzed the incidence of aortic events during pregnancy and puerperium.
ResultsThere were 133 pregnancies in 89 women with MS (8 women with prior aortic surgery). There were no maternal deaths, but 5 women had aortic events during the third trimester and puerperium (type A dissections in 2, type B dissection in 1, and significant [≥ 3mm] aortic growth in 2). The aortic event rate was 3.7%. Pregestational aortic diameter≥ 40mm showed a nonsignificant association with aortic events (P=.058). Fetal mortality was 3% and 37.6% of births were cesarean deliveries.
ConclusionsWomen with MS have an increased risk of aortic events during pregnancy, especially in the third trimester and postpartum period. Patients with MS and aortic diameters ≥40mm should be assessed in experienced centers for prophylactic aortic surgery before pregnancy. It is important to provide early diagnosis, prepregnancy study of the aorta, beta-blocker administration, and close monitoring during pregnancy, especially during the last trimester and postpartum.
Keywords
Marfan syndrome (MS) is the most common genetic disease associated with connective tissue defects.1–4 Aortic involvement (and aortic root involvement in particular) is the main prognostic factor, as it places patients at an increased risk of progressive dilation, rupture, or dissection. Aortic events are the main cause of mortality and reduced life expectancy in MS.5–7
The risk of aortic complications in MS increases during pregnancy due to hemodynamic and hormonal changes associated with increased cardiac output and estrogen/progesterone levels and consequently increased cardiovascular stress.8,9
Recent studies of pregnancy in MS have reported aortic dissection rates of 3% to 8%.10,11 In this setting, pregnant women have a 5- to 23-fold increased risk of dissection compared with nonpregnant women12,13 and a 20 000-fold increased risk compared with the general population.14 Size of the aortic diameter is the main risk factor, but even women with an aortic root diameter <40mm have a 1% risk of dissection.15,16
Current European guidelines recommend aortic surgery before pregnancy in women with an aortic diameter >45mm17 or 40mm to 45mm in the presence of risk factors (family history of early aortic dissection or rapid growth). The American College of Cardiology/American Heart Association guidelines, by contrast, recommend elective aortic surgery before pregnancy in women with an aortic diameter >40mm.18
The above indications, however, are based on clinical experience from case series, observational studies, and reviews.19–21 In addition, more than 50% of the women in these studies were diagnosed with MS during or after pregnancy and consequently did not benefit from the latest prevention and medical treatment recommendations.22,23
In this article, we present our multicenter experience with the management of MS in dedicated MS units and analyze the occurrence of aortic events during pregnancy and postpartum.
MethodsObservational, retrospective study of patients diagnosed with MS based on the Ghent criteria and genetic studies. We included patients managed at a dedicated MS unit at Hospital Universitario Puerta de Hierro in Madrid and Hospital Universitario Vall d’Hebron in Barcelona. These units offer full family screening and diagnostic services as well as multidisciplinary care involving periodic follow-up and current prevention and treatment measures (administration of beta-blockers or losartan, relative limitation of physical activity, periodic echocardiographic assessments and computed tomography (CT)/magnetic resonance (MR) angiography, and preventive surgery in patients with an aortic diameter ≥50mm (or ≥45mm if there are risk factors).24
The study was approved by the ethics committee at our hospital and all patients gave their written informed consent for the retrospective analysis of their data.
PatientsBetween January 2004 and January 2020, 632 patients (323 women) underwent periodic follow-up at the 2 MS units studied. The main clinical characteristics collected were maternal age, personal and family medical history, prepregnancy and peripartum aortic diameters, medication during pregnancy, delivery route, and peripartum complications, including aortic events.
Aortic eventsAortic events were defined as aortic dissection or rapid aortic root growth (≥3mm) requiring aortic surgery during pregnancy or the postpartum period.
Echocardiographic and CT/MR angiographic data on peripartum aortic diameters were systematically collected.
Echocardiographic aortic diameter measurements were made by measuring the aortic root (at the sinuses of Valsalva and sinotubular junction) and ascending aorta in diastole, perpendicular to the long axis of the aorta using the edge to leading edge technique in the parasternal long-axis and apical 5-chamber views.
The maximum orthogonal diameters of the aortic segments were measured by CT/MR angiography.
Follow-up during pregnancyAll pregnant women were advised to take beta-blockers and, where applicable, to stop taking losartan. Echocardiographic assessments were performed every 4 to 8 weeks depending on the prepregnancy aortic diameter (women with a diameter >40mm were assessed every 4 weeks). Monthly clinical check-ups were held up to the third month postpartum. After this period, women continued with their regular follow-up visits at the MS unit every 6 to 12 months.
Statistical analysisContinuous variables are expressed as mean ±standard deviation or median [interquartile range] depending on whether the data were normally or nonnormally distributed. Qualitative variables are expressed as percentages. To investigate factors associated with an increased risk of aortic events during pregnancy and the postpartum period, continuous variables were compared using the t test or Mann-Whitney U test, while categorical variables were compared using the chi-square test or Fisher exact test.
Actuarial survival was calculated using the Kaplan-Meier method. Significance was set at P<.05. Data were stored and analyzed in SPSS v.23.0 (SPSS Inc, USA).
ResultsPatientsOf the 323 women followed at the MS units during the study period, 89 (27.5%) had at least 1 pregnancy (133 pregnancies in total).
The clinical characteristics and results of the prepregnancy imaging tests are shown in table 1. The mean age of the women was 28.4±12.5 years and 8 (9%) had had previous aortic surgery (7 of the interventions were elective and 1 was to treat dissection). Over half (52.8%) of the women were on beta-blockers, losartan, or both before deciding to become pregnant. Overall, 82% of women were treated with beta-blockers during pregnancy and the postpartum period. The mean prepregnancy aortic root diameter was 36.5±6.1mm; 81.1% of women had a diameter <40mm.
Prepregnancy clinical characteristics and imaging findings (transthoracic echocardiography, computed tomography, magnetic resonance)
| Patients (n=89) | |
|---|---|
| Age, y | 28.4±12.5 |
| Body surface area, m2 | 1.8±0.4 |
| Medical treatment before pregnancy | |
| None | 42 (47.2) |
| Atenolol | 24 (26.9) |
| Losartan | 19 (21.4) |
| Combined (atenolol + losartan) | 4 (4.5) |
| Medical treatment during pregnancy | |
| None | 16 (18) |
| Atenolol | 62 (70) |
| Metoprolol | 11 (12) |
| Previous aortic dissection | 4 (4.5) |
| Type A | 1 (25) |
| Type B | 3 (75) |
| Previous aortic surgery | 8 (9) |
| Aortic root | 6 (71.4) |
| Thoracic aorta | 1 (14.3) |
| Root/ascending aorta/arc | 1 (14.3) |
| Previous nonaortic surgery | 2 (2.2) |
| Family history of Marfan syndrome | 64 (72) |
| Genetic study | 57 (64) |
| Left ventricular ejection fraction <50% | 88 (98.9) |
| Diameter of Valsalva sinuses (mm-mm/m2) | 36.5±6.1-20.4±4.7 |
| Diameter <40mm | 73 (81.1) |
| Diameter ≥40mm | 16 (18.9) |
| Ascending aorta diameter, mm | 28.5±6.3 |
| Aortic arc diameter, mm | 22.2±5.0 |
| Ascending aorta diameter, mm | 17.2±5.2 |
| Aortic valve regurgitation ≥II | 5 (5.6) |
| Bicuspid aortic valve | 4 (4.5) |
| Mitral prolapse | 25 (28.1) |
| Patent foramen ovale | 9 (10.3) |
Values are expressed as No. (%) or mean±standard deviation.
Five women experienced an aortic event (2 type A dissections, 1 type B dissections, and 2 rapid increases in aortic root diameter [≥3mm]). This corresponds to an aortic event rate of 3.7% (table 2).
Patient characteristics during the peripartum period
| Patients (n = 89) | |
|---|---|
| Pregnancies, No. | 133 |
| 1 | 55 |
| 2 | 26 |
| 3 | 6 |
| 4 | 2 |
| Aortic events | 5 (3.7) |
| Type A aortic dissection | 2 (40) |
| Type B aortic dissection | 1 (20) |
| Rapid aortic growth | 2 (40) |
| Maternal deaths | 0 |
| Fetal deaths | 4 (3.0) |
| Delivery | |
| Cesarean | 50 (37.6) |
| Vaginal | 83 (62.4) |
| Neonatal complications | 17 (12.8) |
| Low weight | 14 (82.3) |
| Fetal distress | 3 (17.7) |
All values are expressed as No. (%), unless otherwise indicated.
Four of the 5 events (80%) occurred in women with an aortic diameter <45mm during the third trimester or postpartum (table 3).
Characteristics of patients who experienced aortic events during pregnancy
| Age, y | Type of aortic event | Previous aortic event | Time of event | Aortic rootAscending aortaAortic arcDescending aorta, absolute value (mm)/indexed value (mm/m2) | Medical treatment | Delivery | Treatment |
|---|---|---|---|---|---|---|---|
| 40 | Type A aortic dissection | Type B aortic dissection | Third trimester (30 wk) | 43/26.128/16.919/11.516/9.7 | β-blockers | Delayed cesarean section | Emergent Bentall procedurea |
| 30 | Type A aortic dissection | None | Third trimester (32 wk) | 37/20.526/14.422/12.219/10.5 | None | Cesarean section | Emergent Bentall procedure |
| 31 | Aortic dissection, type B | None | Postpartum (Third week) | 45/24.724/13.225/13.736/18.8 | β-blockers | Cesarean section | Conservative treatmentDavid procedure,b 6 mo later |
| 32 | Rapid aortic growth | None | Third trimester (34 wk) | 39/21.1 → 45/24.329/15.621/11.317/9.2 | β-blockers | Cesarean section | Elective David procedure, 4 mo later |
| 42 | Rapid aortic growth | No | Third trimester (34 wk) | 42/22.1 → 47/24.728/15.724/12.619/10 | β-blockers | Cesarean section | Elective David procedure, 3 mo later |
None of the women had a history of aortic surgery and just 1 of the women who experienced type A aortic dissection had a history of type B dissection.
The mean aortic root diameter after pregnancy was 37.2±6.6mm (increase of 0.7±0.16mm).
Just 2 preoperative clinical variables showed a tendency towards an increased risk of aortic complications during pregnancy: an aortic diameter ≥40mm (P=.058) and an aortic diameter indexed to body surface area ≥25mm/m2 (P=.067).
Follow-upAll the patients underwent clinical and echocardiographic follow-up (mean duration, 8.1±2.6 years; median, 8.6 years).
Eighteen patients underwent elective surgery: 17 valve-sparing aortic root replacements and 1 descending aortic replacement to treat chronic type B dissection. The mean aortic root diameter in these patients was 47.7±3.8mm.
Two patients died during follow-up: a 55-year-old woman with a history of elective mitral valve replacement and a 53-year-old woman with a history of mitral valve and aortic root replacement to treat acute type A dissection. Both patients died of stroke: the first 9 years after pregnancy and the second 10 years after pregnancy.
Five women experienced aortic dissection (3 type A dissections and 2 type B dissections) during follow-up.
Pregnant women were significantly younger than those who did not become pregnant, and a higher percentage were on medical treatment for MS and had a history of elective aortic surgery (table 4).
Univariate analysis comparing pregnant and nonpregnant women with Marfan syndrome
| Pregnant women (n=89) | Pregnant women (n=234) | P | |
|---|---|---|---|
| Age, y | 28.4±12.5 | 37.2±14.5 | .04 |
| Body surface area, m2 | 1.8±0.4 | 1.7±0.4 | .95 |
| Previous treatment | 47 (53.1) | 107 (45.7) | .06 |
| Valsalva sinus diameters, mm | 36.5±6.1 | 36.3±6.9 | .97 |
| Aortic dissection | 8 (8.9) | 16 (6.8) | .08 |
| Elective aortic dissection | 18 (20.2) | 36 (14.9) | .05 |
| Death during follow-up | 2 (2.2) | 6 (2.5) | .45 |
The data are expressed as No. (%) or mean ± standard deviation.
No significant differences were observed for the combined endpoint of aortic events, elective aortic surgery, and death between women who did not experience aortic complications during pregnancy and those who did not become pregnant (29.8% vs 24.8%, P=.42).
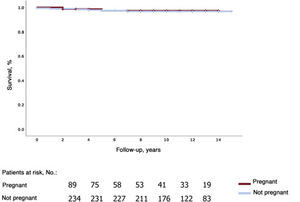
The respective 1-, 5-, and 10-year survival rates were 100%, 99.1%±0.9%, and 96.3%±2.9% in the group of pregnant women and 99.5%±0.5%, 97.4%±1.2, and 96.8%±1.3% in the group of nonpregnant women (figure 1).
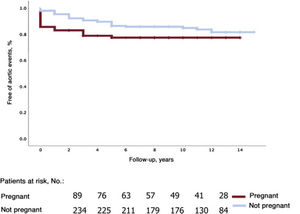
The corresponding rates for survival free of aortic events (aortic dissection and elective surgery) were 85.5%±4%, 77.3%±4.8%, and 77.3%±4.8% for pregnant women and 97.9%±1%, 89.5%±2.2%, and 84.8%±2.7% for nonpregnant women (figure 2).
DiscussionThis is the first Spanish study and one of the largest series to date to analyze the risk of peripartum aortic events in women with MS followed up at dedicated units and managed according to the latest prevention and treatment recommendations.24
Little has been published on the risk of aortic events during pregnancy in MS, and most studies have focused on women who were not managed at a dedicated unit before becoming pregnant. Studies have also largely focused on the risk of aortic dissection,11,13,23,25 with little attention paid to other clinically relevant aortic events, such as aortic growth during pregnancy.
The overall rate of aortic events during pregnancy in our series of MS patients was 3.7% (2 type A dissections, 1 type B dissection, and 2 cases of rapid aortic growth). The specific dissection rate was 2.2%, which is lower than most rates reported in previous studies (1.9%-7.9%).11–13,23,25 This lower rate might be due to close monitoring at dedicated MS units and a higher rate of beta-blocker use before and during pregnancy (82%). In a multicenter study of 151 pregnant women with MS in the United Kingdom, Cauldwell et al.25 reported that 64.2% of women had been treated with beta-blockers and 1.9% experienced aortic dissection. By contrast, Lind et al.26 reported 5 dissections in 44 women with 147 pregnancies treated with beta-blockers. In the GenTAC (Genetically Triggered Aortic Aneurysms and Cardiovascular Conditions) registry, 4.4% of pregnant women with MS experienced an aortic event and fewer than 50% were treated with peripartum beta-blockers.13
Mean aortic diameter growth during pregnancy was 0.7mm in our series, with faster growth observed in the third trimester and postpartum period. Renard et al.27 also found that aortic diameters grew fastest in the postpartum period and then stabilized. These findings highlight the importance of continued surveillance of women with MS during the peripartum period.
The low rate of aortic events in our series of MS patients prevented us from identifying risk factors for complications during pregnancy. We only observed a tendency towards an increased risk in women with a prepregnancy aortic diameter > 40mm: 18.7% of these women experienced an aortic event vs just 2.7% of those with a diameter <40mm (odds ratio=3.32; P=.058).
The 5 cases of aortic dissection reported in the UK multicenter study occurred in women with an aortic diameter <45mm.25 In a retrospective review of 852 women with MS (1112 pregnancies), Kim et al.11 reported 88 aortic dissections, and 63% of the women had an aortic diameter ≥40mm.
The above findings are consistent with the US guideline recommendation to perform preventive aortic surgery in women with MS with a prepregnancy aortic diameter >40mm.18 The European Guidelines, by contrast, establish a threshold of 45mm.17
Of note, even women who do not have a dilated aortic root before they become pregnant, or who undergo preventive aortic root surgery, have an increased risk of dissection during pregnancy. Cauldwell et al.25 reported a rate of 3.4% for type B aortic dissections in pregnant women with MS who had undergone preventive aortic root surgery. Prepregnancy MR/CT angiography should be performed in all women with MS.
Delivery route is not a risk factor for aortic events. In our series, 62% of the deliveries were vaginal, supporting previous reports of the safety of vaginal delivery in women with MS.11,23,25,28 Cesarean section should be performed when deemed necessary by the treating obstetrician and in patients with a history of aortic events or an aortic diameter ≥40mm.
In line with previous studies, all the aortic events in our series occurred in the third trimester or postpartum. This is consistent with the physiology of pregnancy, where cardiovascular demands peak at 32 weeks and remain high for the first 3 months postpartum.8,29 Habashi et al.30 showed a possible association between risk of aortic events and oxytocin levels, which increase in the last few months of pregnancy and remain high during breastfeeding. Again, these findings highlight the importance of follow-up and close monitoring of pregnant women with MS, especially in the third trimester and postpartum.
Study limitationsThis study has the statistical limitations inherent to its observational, retrospective design. We did not systematically monitor peripregnancy blood pressure levels and were therefore unable to investigate links with aortic events. Nonetheless, because all the patients were managed in dedicated MS units, their data were collected prospectively and there were no losses to follow-up. Although this is one of the largest series to date of pregnant women with MS, our statistical analyses were limited by the small number of events. Larger, prospective multicenter studies could help identify risk factors for aortic events during pregnancy in MS.
ConclusionsPregnancy is associated with an increased risk of aortic events in women with MS, particularly in the third trimester and postpartum period. The option of preventive surgery for women with an aortic diameter ≥40mm should be evaluated in hospitals with experience in this field. It is important to provide an early diagnosis, prepregnancy assessment of the entire aorta, beta-blocker administration, and close monitoring during pregnancy, especially during the last trimester and postpartum period.
- –
Pregnant women with MS have an increased risk of aortic complications, but current clinical experience is based on case series, observational studies, and reviews. In addition, more than 50% of the women in these studies were not diagnosed with MS before they became pregnant and therefore did not benefit from management at a dedicated MS unit and the latest prevention and medical treatment recommendations.
- –
This is the first Spanish study and one of the largest series to date to analyze the risk of aortic events (acute aortic dissection and rapid aortic growth) in pregnant women with MS managed at a dedicated MS unit. Although the rate of acute aortic dissection was relatively low (2.2%), we observed a tendency towards an increased risk in women with a prepregnancy aortic diameter >40mm. It is important to provide an early diagnosis, prepregnancy assessment of the entire aorta, beta-blocker administration, and close monitoring during pregnancy, especially during the last trimester and postpartum period.
No funding of any nature was received for the preparation of this manuscript.
Authors’ ContributionsC.E. Martín: conceptualization, data collection, design, analysis, and original draft preparation. A. Evangelista and G. Teixidó: data collection and review of manuscript content. S. Villar, S. Serrano-Fiz, V. Ospina, S. Mingo, V. Moñivas, D. Martínez, and J. Villarreal: review of intellectual content. A. Forteza: conceptualization, interpretation, and review of intellectual content.
Conflicts of InterestThere are no conflicts of interest.