To evaluate by cardiovascular magnetic resonance those factors related to the amount of salvaged myocardium after a myocardial infarction and its value in predicting adverse ventricular remodeling.
MethodsOne hundred eighteen patients admitted for a first ST elevation myocardial infarction (primary angioplasty, 65 patients; a pharmacoinvasive strategy, 53 patients) underwent magnetic resonance (6 [5-8] days and 6 months; n=83). The myocardial salvage index was quantitatively assessed as the percentage of area at risk (T2-weighted sequences) not showing late enhancement.
ResultsMyocardial salvage index >31% (median) was associated with a shorter time to reperfusion (153min vs 258min), a lower rate of diabetes (12% vs 32%), shorter time to magnetic resonance, and better cardiovascular parameters (P<.05 for all analyses). There were no significant differences depending on the reperfusion method. In a logistic regression analysis, delayed reperfusion (odds ratio=0.42 [0.29-0.63]; P<.0001), diabetes (odds ratio=0.32 [0.11-0.99]; P<.05) and a longer time to the performance of magnetic resonance (odds ratio=0.86 [0.76-0.97]; P<.05) were independently related to a lower probability of a myocardial salvage index >31%. Predictors of increased left ventricular end-systolic volume at 6 months were the number of segments showing an extent of transmural necrosis >50% (odds ratio =1.51 [1.21-1.90]; P<.0001) and left ventricular end-systolic volume at one week (odds ratio=1.12 [1.06-1.18]; P<.0001).
ConclusionsCardiovascular magnetic resonance enables the quantification of the salvaged myocardium after myocardial infarction. The celerity with which reperfusion therapy is administered constitutes its most important predictor. The possible effect of a delay in the performance of magnetic resonance on myocardial salvage needs to be confirmed. Salvaged myocardium does not improve the value of magnetic resonance for predicting adverse remodeling.
Keywords
.
IntroductionCardiovascular magnetic resonance (CMR) has been established as a valuable tool for the characterization of postinfarction necrosis.1 Moreover, in the early phases, both acute ischemia and reperfusion lead to the formation of myocardial edema that is detectable on T2-weighted sequences.2 In histological studies, the area of the edema has been shown to be comparable to the area of myocardium at risk following experimental coronary occlusion.3 Thus, it is possible to quantify the difference between this area and the area of late gadolinium enhancement (extent of necrosis), referred to as salvaged myocardium,4 the achievement of which is the purpose of all the postinfarction reperfusion strategies. CMR is a unique technique because it enables retrospective quantification, given that the edema persists over time after the acute event.5.
Logically, a delay in reperfusion has an influence on the amount of myocardium salvaged, although the literature is not conclusive with respect to the importance and the limits of this impact.6, 7 Thus, the main objective of this report is to analyze, using CMR, the factors that determine the amount of myocardium salvaged in patients who have undergone reperfusion following ST-segment elevation myocardial infarction (STEMI). The secondary objective is to assess the influence of the salvaged myocardium on left ventricular (LV) remodeling occurring early and observed at 6 months, and its additional predictive value with respect to other CMR parameters..
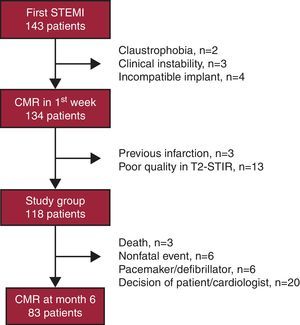
Methods Study GroupThis prospective study included patients who, between February 2008 and August 2010, were admitted consecutively to a tertiary hospital with a first STEMI, and underwent primary percutaneous coronary intervention (PCI) or were treated with a pharmacoinvasive strategy (thrombolysis during the first 12h after the onset of symptoms, followed systematically by PCI at least 3h later; revascularization was carried out in those patients with severe residual lesions),8 and evaluated by means of CMR prior to hospital discharge and 6 months later. The exclusion criteria are shown in Figure 1..
Figure 1. Flow chart representing the patients included in the study. CMR, cardiovascular magnetic resonance; STEMI, ST-segment elevation myocardial infarction; STIR, short tau inversion recovery.
Data collection included the major clinical and laboratory variables, plus the risk factors, Killip class at admission, percent ST-segment resolution 90min after reperfusion, and peak troponin I levels (ng/mL). We also recorded the times elapsed between the onset of pain and reperfusion, from onset of pain to arrival at the emergency service, and from arrival to reperfusion. Medication was administered at the discretion of the clinical cardiologist. An expert observer determined the Thrombolysis in Myocardial Infarction (TIMI) flow grade prior to and after the PCI using a standard software package (Integris HM3000, Philips; Best, The Netherlands)..
The protocol received the approval of the local ethics committee, and written informed consent was obtained from all the patients..
Cardiovascular Magnetic ResonanceCMR (1.5 T Magnetom Sonata® magnetic resonance system, Siemens; Erlangen, Germany) was performed prior to discharge from the hospital and 6 months later according to the protocol used in our center.2, 9 All the images were acquired by means of a phased-array body surface coil during repeated breath holds, using electrocardiographic triggering..
The cine images were acquired in 2-chamber, 3-chamber, 4-chamber, and short-axis views from the mitral valve to the apex, using steady-state free precession imaging..
For the detection of the area at risk, segmented T2-weighted short tau inversion recovery (T2-STIR) turbo spin echo sequences were employed to acquire images in projections identical to those of the cine images, in mid-diastole..
The detection of myocardial necrosis was carried out at least 10min after the administration of gadolinium at a dose of 0.1 mmol/kg body weight (gadopentetate dimeglumine; Magnevist®) in projections identical to those of the cine images, using segmented inversion recovery sequences and canceling out the signal from the healthy myocardium..
Analysis of the Cardiovascular Magnetic Resonance ImagesTwo experienced observers who were blinded to the clinical data of the patients analyzed the studies using the QMASS MR 6.1.5 software package (Medis Medical; Leiden, The Netherlands)..
We quantified the LV volume (mL/m2), LV ejection fraction using Simpson's method (%), and LV mass (g/m2) by manually tracing the endocardial and epicardial borders in all the short-axis cine images. The 17-segment model was employed.10.
The area of myocardium at risk was quantified in the T2-STIR images as that having a signal intensity≥2 standard deviations above the signal from remote noninfarcted myocardium (% LV mass). Intramyocardial hemorrhage was considered to be present when there was a region of low signal intensity surrounded by a region of hyperintensity.11.
The area of necrosis was quantified in the delayed enhancement sequences as that in which the signal intensity was ≥2 standard deviations above the signal from remote noninfarcted myocardium (% LV mass). We also quantified the number of segments with a degree of transmurality over 50%. Microvascular obstruction was defined as a region displaying a lack of signal situated within a region with an enhanced signal.8.
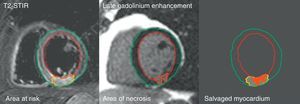
The operators reviewed all the measurements and corrected them manually, if necessary. Salvaged myocardium was defined as the difference between the area at risk and the area of necrosis, expressed as the percent LV mass or percent area at risk (myocardial salvage index [MSI]) (Figure 2)..
Figure 2. Images of the area at risk (T2-STIR), area of necrosis (late gadolinium enhancement), and salvaged myocardium resulting from the differences between the two. STIR, short tau inversion recovery.
Six months after STEMI, CMR was repeated and all the indices were reevaluated. We analyzed the reproducibility of the measurements in a subgroup of 20 patients, and it was greater than 95% for all the CMR variables. Specifically, there was interobserver agreement—intraclass correlation coefficient, 0.96 (95% confidence interval [95%CI], 0.91-0.99)—and intraobserver agreement (0.98 [95%CI, 0.95-0.99]) with respect to the quantification of the area at risk in T2-weighted sequences..
Statistical AnalysisThe Kolmogorov-Smirnov test was employed to assess the normal distribution of the variables. Consequently, the data are expressed as mean (standard deviation) or median [25th-75th percentiles], and parametric or nonparametric tests were used for univariate analysis, depending on the case..
The minimum amount of myocardium that must be salvaged in order to achieve a significant improvement in the prognosis is unknown. Thus, in the interests of practicality and by dividing the MSI data into quartiles, we constructed a receiver operating characteristic (ROC) curve to calculate the maximum length of time that can be allowed between the onset of pain and reperfusion in order to achieve an MSI>25th percentile.Using multivariate logistic regression, we analyzed the independent predictors of an MSI greater than the median, as well as adverse LV remodeling. The clinically important variables with a value of P<.10 were retained in the model. A value of P<.05 was considered to indicate statistical significance..
ResultsCMR was performed during the first week in 134 patients. Three were excluded due to previous infarction and 13 because of the poor quality of the T2-STIR images (Figure 1). Thus, the final group consisted of 118 patients. In all, 65 patients (55%) underwent primary PCI and 53 (45%) a pharmacoinvasive strategy..
CMR was repeated 6 months (181 [11] days) later in 83 patients (70%)..
The mean salvaged myocardium and mean MSI were 9.8%±8.1% and 35 (23), respectively..
The clinical and angiographic characteristics of the overall study group and of two subgroups, MSI greater than the median and MSI less than or equal to the median (31%), are shown in Table 1. The prevalence of diabetes was higher in the patients with a lower MSI. There were no other significant differences in the baseline characteristics or the medical treatment received, nor were differences observed in the magnitude of the MSI according to the initial reperfusion strategy (primary PCI, 36 (23), vs pharmacoinvasive therapy, 35 (23); P=.9). The presence of a lower MSI was associated with a poorer Killip class, higher peak troponin I level, and lower incidence of ST-segment resolution..
Table 1. Characteristics of the Patients Grouped According to the Median Myocardial Salvage Index
| Overall group | MSI>median (31%) | MSI≤median (31%) | P | |
| Patients | 118 | 59 | 59 | |
| Age, years | 59 ±13 | 60 ±12 | 58 ±13 | .5000 |
| Men | 89 (75) | 43 (73) | 46 (76) | .4000 |
| Body surface area, m2 | 1.8±0.4 | 1.9±0.2 | .1000 | |
| Diabetes | 22 (19) | 7 (12) | 15 (32) | <.0500 |
| Hypertension | 68 (58) | 33 (51) | 35 (66) | .1000 |
| Dyslipidemia | 52 (44) | 28 (48) | 24 (41) | .6000 |
| Smoker | 64 (54) | 29 (49) | 35 (59) | .4000 |
| Number of risk factors | 1.9±0.9 | 1.7±0.8 | 2±1 | .1000 |
| Previous coronary intervention | 7 (6) | 4 (6.8) | 3 (5.2) | .5000 |
| Creatinine, mg/dL | 1±0.4 | 1±0.3 | 1±0.6 | .7000 |
| Peak troponin I, ng/mL | 83±90 | 60±48 | 107±114 | <.0100 |
| Pain-to-reperfusion time, min | 180 [139-300] | 153 [113-184] | 258 [180-420] | <.0001 |
| Pain-to-ED door time, min | 111 [71-158] | 90 [60-120] | 120 [90-300] | <.0001 |
| ED door-to-reperfusion time, min | 60 [32-110] | 51 [30-80] | 74 [46-128] | <.0100 |
| Delay in CMR, days | 6 [5-8] | 6 [5-7] | 7 [5-9] | <.0500 |
| Killip class | ||||
| I | 103 (88) | 57 (97) | 46 (79) | <.0500 |
| II | 12 (9) | 2 (3) | 10 (16) | |
| III | 2 (2) | 0 | 2 (3) | |
| IV | 1 (1) | 0 | 1 (2) | |
| ADA involvement | 58 (49) | 23 (39) | 35 (58) | <.0500 |
| ST-segment resolution | 85±22 | 90±16 | 79±25 | <.0100 |
| Primary PCI strategy | 65 (55) | 30 (49) | 35 (56) | .6000 |
| Pharmacoinvasive strategy | 53 (45) | 30 (51) | 23 (39) | .3000 |
| Initial TIMI flow rate | .1000 | |||
| 0 | 62 (53) | 31 (54) | 31 (52) | |
| 1 | 9 (7) | 1 (2) | 8 (12) | |
| 2 | 11 (9) | 4 (7) | 7 (12) | |
| 3 | 36 (31) | 24 (37) | 12 (24) | |
| Final TIMI flow rate | .2000 | |||
| 0 | 2 (2) | 1 (2) | 1 (2) | |
| 1 | 2 (2) | 1 (2) | 1 (2) | |
| 2 | 11 (8) | 3 (3) | 8 (14) | |
| 3 | 103 (88) | 55 (93) | 48 (82) | |
| Antiplatelet agents | 113 (98) | 56 (98) | 57 (99) | .9000 |
| Anticoagulants | 8 (7) | 3 (4) | 5 (10) | .3000 |
| Anti-GPIIb/IIIa | 44 (37) | 22 (38) | 22 (36) | .9000 |
| Beta blockers | 81 (69) | 40 (68) | 41 (70) | .9000 |
| ACE inhibitors | 91 (77) | 46 (79) | 45 (76) | .8000 |
| ARB | 18 (15) | 11 (20) | 7 (14) | .7000 |
| Statins | 98 (83) | 50 (85) | 48 (80) | .8000 |
ACE, angiotensin-converting enzyme; ADA, anterior descending artery; anti-GPIIb/IIIa, glycoprotein IIb/IIIa inhibitors; ARB, angiotensin receptor blockers; CMR, cardiovascular magnetic resonance; ED, emergency department; MSI, myocardial salvage index; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Data are expressed as no (%), mean±standard deviation, or median [interquartile range].
Anterior descending coronary artery involvement was more common among the patients with an MSI less than or equal to the median. This may be influenced by the fact that the area at risk that is dependent on this vessel is larger than in the remainder of the patients (38 [15] vs 23 [12]; P<.0001). Likewise, the patients with a higher MSI tended to have a higher TIMI flow grade at the beginning and end of the catheterization..
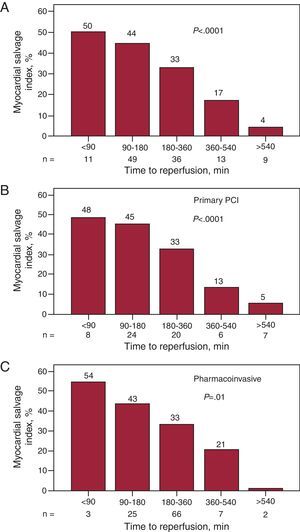
Relationship Between Salvaged Myocardium and the Delay in ReperfusionThe median time between the onset of pain and reperfusion in our patients was 3h (180 [139-300] min), and we found that both the total time and the partial times (pain-to-door time and door-to-reperfusion time, where “door” was defined as arrival in the emergency department) were significantly longer in the patients with a lower MSI, as was the delay to the performance of CMR (Table 1). When we stratified the delay to reperfusion, we observed that its increase was associated with a progressively lower MSI (Figure 3). This trend was significant both when we considered the overall group and when the group was divided according to the reperfusion strategy employed..
Figure 3. Bar graphs showing the myocardial salvage index over time from the onset of pain to reperfusion. A, overall study group. B, patients who underwent primary percutaneous coronary intervention. C, patients treated with a pharmacoinvasive strategy. The P value for trend is significant in every case. PCI, percutaneous coronary intervention.
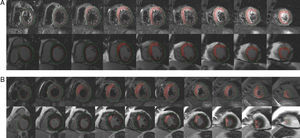
To meet the objective of achieving an MSI above the 25th percentile (16.3%), the pain onset-to reperfusion time should not be longer than 5h and 35min: area under the ROC curve, 0.77 (0.66-0.88) (P<.0001); sensitivity, 59%; specificity, 92%; positive predictive value, 0.88; and negative predictive value, 0.75. Figure 4 shows the images of the area at risk and the area of necrosis in 2 patients in our series..
Figure 4. Areas of hyperintensity representative of the area of risk (T2-STIR images, top) and the area of necrosis (late gadolinium enhancement images, bottom). Patient A was reperfused 340min after the onset of pain and has an myocardial salvage index of 16%. Patient B was reperfused 115min after the onset of pain and has an myocardial salvage index of 82%. STIR, short tau inversion recovery.
Predictors of Salvaged MyocardiumThe variable with the strongest independent relationship to an MSI greater than the median was the total time to reperfusion (Table 2). The other variables included were diabetes and a longer delay in the performance of CMR during the hospital stay. When we analyzed only the patients in whom CMR was carried out prior to day 8 (n=95), this variable ceased to have predictive value in both the univariate and the multivariate analyses, but the pain onset-to-reperfusion time and diabetes continued to be independent predictive variables..
Table 2. Multivariate Analysis
| OR (95%CI) | P | |
| Independent predictors of MSI>median (31%) a | ||
| Total time to reperfusion | 0.42 (0.29-0.63) | <.0001 |
| Diabetes | 0.32 (0.11-0.99) | <.0500 |
| Time to CMR | 0.86 (0.76-0.97) | <.0500 |
| Independent predictors of MSI>median (31%) b | ||
| Total time to reperfusion | 0.42 (0.28-0.64) | <.0001 |
| Diabetes mellitus | 0.25 (0.07-0.99) | <.0500 |
95%CI, 95% confidence interval; CMR, cardiovascular magnetic resonance; MSI, myocardial salvage index.
a The model includes diabetes mellitus, total time to reperfusion, time to CMR, Killip class, percent ST-segment resolution, and initial Thrombolysis in Myocardial Infarction (TIMI) flow rate.
b Includes only patients who underwent CMR in the first 8 days after the infarction (n=95). The model includes diabetes mellitus, total time to reperfusion, Killip class, and percent ST-segment resolution.
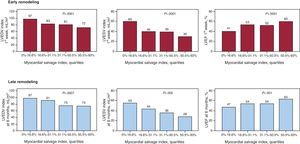
We found an association between an MSI value less than or equal to the median and less favorable values for the major CMR variables during the first week (Table 3). Six months later, this association persisted, although there was no longer evidence of edema, microvascular obstruction, or intramyocardial hemorrhage in any case. Figure 5 shows the indices of early and late LV remodeling according to the MSI, divided into quartiles, and there are significant differences in every case. When we considered increased end-systolic LV volume in the 6th month (in accordance with the reference values for age, sex, and body surface12) as a dependent variable, the independent predictive values were end-systolic LV volume in the first week and the number of segments with >50% necrosis in the first week (Table 4). Thus, in our series, the MSI did not provide additional value for the prediction of adverse remodeling (P=.4)..
Table 3. Characteristics of Cardiovascular Magnetic Resonance of the Patients Grouped According to the Median Myocardial Salvage Index
| Overall group | MSI>median (31%) | MSI≤median (31%) | P | |
| CMR 1st week | ||||
| Patients, no. | 118 | 59 | 59 | |
| End-diastolic volume, mL/m2 | 83 (23) | 76 (18) | 90 (26) | .0010 |
| End-systolic volume, mL/m2 | 42 (22) | 34 (14) | 50 (25) | <.0001 |
| Ejection fraction, % | 51 (13) | 56 (11) | 47 (13) | <.0001 |
| LV mass, g/m2 | 75 (17) | 71 (14) | 78 (20) | <.0500 |
| Area at risk, % LV mass | 30 (16) | 29 (14) | 31 (16) | .2000 |
| Area of necrosis, % LV mass | 21 (15) | 13 (9) | 28 (15) | <.0001 |
| Number of segments with necrosis s>50% | 3.8 (3.0) | 2.4 (2.2) | 5.1 (3.0) | <.0001 |
| Microvascular obstruction, % LV mass | 3.6 (5.4) | 1.8 (3.7) | 5.3 (6.2) | .0010 |
| Intramyocardial hemorrhage, % LV mass | 2.5 (3.9) | 1.1 (2.3) | 3.7 (4.8) | .0010 |
| CMR 6th month | ||||
| Patients, no. | 83 | 43 | 40 | |
| End-diastolic volume, mL/m2 | 84 (26) | 75 (22) | 94 (28) | .0010 |
| End-systolic volume, mL/m2 | 40 (24) | 32 (15) | 49 (29) | .0010 |
| Ejection fraction, % | 55 (14) | 59 (11) | 51 (15) | <.0100 |
| LV mass, g/m2 | 73 (20) | 70 (19) | 76 (20) | .1000 |
| Area of necrosis, % LV mass | 17 (10) | 14 (11) | 20 (8) | .0500 |
CMR, cardiovascular magnetic resonance; LV, left ventricle; MSI, myocardial salvage index.
Data are expressed as mean (standard deviation), unless otherwise indicated.
Figure 5. Bar graphs representing the early (top) and late (bottom) indices of left ventricular remodeling according to the quartiles of the myocardial salvage index. Increased myocardial salvage index values are associated with significantly better values for all 3 indices. LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume.
Table 4. Multivariate Analysis. Independent Predictors of Adverse Remodeling at 6 Months (Increased End-Systolic Volume)
| OR (95%CI) | P | |
| End-systolic volume at 1 week, mL/m2 | 1.12 (1.06-1.18) | <.0001 |
| Number of segments with necrosis>50% at 1 week | 1.51 (1.21-1.90) | <.0001 |
95%CI, 95% confidence interval; OR, odds ratio.
This report confirms the importance of CMR as a noninvasive tool for the detection and quantification of salvaged myocardium following reperfusion therapy for STEMI. The facts that it is noninvasive and can be performed retrospectively make it the most suitable technique for the evaluation of any postinfarction reperfusion strategy. We also show that the celerity with which reperfusion therapy is administered and presence of diabetes are the major determinants of the magnitude of its effects. The delay in the performance of CMR can influence the amount of myocardium salvaged. Both the size and transmurality of the infarction and early LV remodeling are superior to the amount of salvaged myocardium as predictors of late adverse remodeling..
At the present time, CMR is the best technique for the accurate measurement of infarction size and transmurality of the necrosis.13 On the other hand, both the infarction and reperfusion produce inflammation, with secondary intracellular and extracellular edema, that increases signal intensity in the T2-weighted images.14 The edematous area has been shown to reflect the perfusion bed of a coronary artery (area at risk) in both reperfused15 and nonreperfused16 infarctions. Given that the edema persists transitorily after acute myocardial infarction, it can be measured retrospectively following revascularization, a circumstance that facilitates its use for clinical purposes.2, 15 Thus, the area at risk includes both the area of the necrosis and the area surrounding the myocardial infarction that is reversibly injured and basically viable, and the salvaged myocardium is obtained by calculating the difference between these two areas..
A number of studies have analyzed the myocardium salvaged after a STEMI by means of CMR.4, 7 These reports have demonstrated that it is a robust method for evaluating the efficacy of reperfusion in a clinical context, and a better indicator of success than the size of the infarction.6 Some studies have credited the salvaged myocardium with having prognostic value with regard to LV remodeling17 or adverse cardiovascular events.7.
There is no general agreement as to the influence of the delay to reperfusion on the amount of salvaged myocardium. Thus, in a pooled analysis performed by Boersma et al.,18 the authors point out that the delay to reperfusion is not as important in primary PCI as in thrombolysis. In a study involving the use of a radioactive agent, Shomig et al.19 found no significant relationship between the time to PCI and infarction size. According to the ischemic wave front theory postulated by Reimer and Jennings,14 ischemic myocardium progresses rapidly toward necrosis during the first few minutes following coronary occlusion, and the process is completed 6h later. Although with variable findings, the published reports6, 7 show that the amount of myocardium salvaged decreases more or less rapidly in accordance with the delay in reperfusion. This trend is maintained in our patients, although a certain amount of salvaged myocardium remains after 6h, a fact that suggests the influence of other factors such as the presence of collateral circulation and a history of ischemic preconditioning.20.
Our study provides information on patients treated with a pharmacoinvasive strategy not represented in other studies which indicates that the influence of time to reperfusion is crucial, regardless of the initial management strategy. This finding agrees with the recommendation of the clinical practice guidelines of the European Society of Cardiology that early pharmacological or mechanical (PCI) reperfusion be carried out within the first 12h after the onset of symptoms in patients with STEMI.21 Recently, we demonstrated that this strategy constitutes an alternative with results similar to those obtained with primary PCI with respect to short- and long-term LV involvement.8.
Another factor predictive of the MSI is diabetes, the influence of which may be related to preexisting microvascular injury and dysfunction in these patients which, in turn, leads to a greater degree of microvascular obstruction following infarction and revascularization.22.
We have verified the value of the time elapsed between the acute event and the performance of CMR as a predictor of the MSI in the overall study group, but this is not the case if we exclude those patients in whom CMR is carried out more than 8 days after the event. The literature does not clearly establish the duration of edema produced after acute ischemic injury and, although its extent is greatest during the first week,23 significant reductions have also been detected prior to day 7,24 a circumstance that may explain the fact that the “real” area at risk is underestimated in patients who undergo late CMR..
With respect to the influence of the salvaged myocardium on adverse LV remodeling, we have found positive17 and negative25 results in the literature. Our report reveals an association between the MSI and a more extensive LV remodeling, but it disappeared after adjustment for the remaining variables. These results agree with the concept that the final infarction size is the parameter with the greatest influence on systolic dysfunction, and consequently on prognosis,25 regardless of the MSI. Thus, further research will be required to clarify the relationship between the MSI and the prognosis. Moreover, in our study, the number of segments with necrosis greater than 50% on visual analysis is an even better predictor of adverse remodeling than the size of the infarction itself. This is probably due to the fact that a single parameter combines information relative to the extension of the infarction and to its transmurality..
Clinical ImplicationsThe present report demonstrates the clinical value of CMR as an alternative noninvasive imaging technique for the quantitative assessment of the reversible and irreversible injury produced in reperfused STEMI. CMR may be the optimal method for evaluating salvaged myocardium since it has a high spatial resolution and can be performed retrospectively in a single test, without interfering with management during the acute phase and without irradiating the patient..
There appears to be little doubt as to the superiority of the MSI as the best parameter for evaluating reperfusion therapies focusing on myocardium with edema but without necrosis, that is, with reversible injury and thus viable. The advantage of an approximation based on adjusting the salvaged myocardium for the area of risk, in contrast to measuring only the area of infarction, lies in the fact that it helps to avoid, in part, the bias produced by differences in the baseline clinical characteristics of the patients26 and by other factors such as collateral flow, the duration of ischemia, and metabolic demands,27 which play a role in the wide variability observed in infarction size..
Our results, which demonstrate the relationship between salvaged myocardium and the time to reperfusion, support the recommendations to revascularize patients with STEMI as soon as possible.21.
We have detected a relationship between the delay in carrying out CMR and the MSI and, thus, the early performance of this study would appear to be advisable. However, the time course of the development of edema and the factors determining its persistence have yet to be fully clarified. Therefore, further studies will be necessary before firm recommendations can be made in this respect..
Study LimitationsPatient management involved a pharmacoinvasive strategy or primary PCI, depending on availability and on the criteria of the clinical cardiologist, and thus this is a nonrandomized study. Therefore, there may be differences in the clinical profile of the patients that have an impact on the results, although as we have seen these differences were small..
The T2-STIR images have a poorer signal-to-noise ratio than most CMR images and, on occasion, result in an erroneous interpretation of the unsuppressed signal of slow flowing blood which, in certain cases, makes it difficult to clearly define the borders of the hyperintense area. Thus, technical improvements are still necessary in images of this type in order for them to achieve the robustness of delayed enhancement images. Research is underway with T2 mapping which may correct some of the limitations of T2 images and could, perhaps in the near future, change this situation.28.
In the multivariate analysis, certain covariates of less importance in clinical terms or that could provide redundant information have been eliminated for the purpose of avoiding an overly adjusted model. Thus, its predictive capacity must be confirmed with larger sample sizes..
ConclusionsCMR is an essential technique for assessing the amount of myocardium salvaged following STEMI. Its noninvasive nature and the fact that it can be performed retrospectively make it the best technique for evaluating any reperfusion strategy after an infarction. The celerity with which reperfusion therapy is received is its most important predictor. There may be a relationship between the delay in carrying out CMR and the amount of myocardium salvaged that must be confirmed in specific studies. The size and transmurality of the infarction are better indicators than salvaged myocardium for predicting adverse remodeling at 6 months..
FundingThe present study was funded by the Instituto de Salud Carlos III, Spain (grant no. PI080128 and a grant from the Heracles Network)..
Conflicts of interestNone declared..
Received 22 November 2011
Accepted 29 January 2012
Corresponding author: Unidad de Imagen Cardiaca (ERESA), Hospital Clínico Universitario de Valencia, Avda. Blasco Ibáñez 17, 46010 Valencia, Spain. jmonmeneu@eresa.com