The risk of stroke in atrial fibrillation is heterogeneous and depends upon underlying clinical conditions included in current risk stratification schemes. Recently, the CHA2DS2-VASc score has been included in guidelines to be more inclusive of common stroke risk factors seen in everyday clinical practice, and useful in defining “truly low risk” subjects. We aimed to assess the usefulness of CHA2DS2-VASc score to give us an additional prognostic perspective for adverse events and mortality among “real world” anticoagulated patients with atrial fibrillation who are often elderly with many comorbidities.
MethodsConsecutive outpatients with permanent/paroxysmal nonvalvular atrial fibrillation with CHA2DS2-VASc≥2 and stabilized oral anticoagulation (international normalized ratio 2.0-3.0) for at least the preceding 6 months were recruited. Patients with CHA2DS2-VASc≥2 were selected. Adverse cardiovascular events including stroke, acute coronary syndrome, or heart failure; major bleeds; and mortality were recorded during more than 2.5-year-follow-up.
ResultsOf 933 patients (93.5%) assessed, 432 were males, median age 76 (71-81) years. After a follow-up of 946 (782-1068) days, 109 patients (11.7%) had adverse cardiovascular events, 80 patients (8.6%) had major bleeds, 101 patients (10.8%) died, and 230 (24.6%) major adverse events (composite end-point). Increasing CHA2DS2-VASc score by 1 point had a significant impact on the occurrence of cardiovascular events (hazard ratio=1.27; 95% confidence interval, 1.13-1.44; P<.001), mortality (hazard ratio=1.36; 95% confidence interval, 1.19-1.54, P<.001); and major adverse events (hazard ratio=1.23; 95% confidence interval, 1.13-1.34; P<.001). CHA2DS2-VASc score was not associated with major bleeding episodes.
ConclusionsAmong high risk atrial fibrillation patients on oral anticoagulation, CHA2DS2-VASc successfully predicts cardiovascular events and mortality, but not major bleeds.
Keywords
.
IntroductionAtrial fibrillation (AF) increases 5-fold the risk for stroke and thromboembolism.1 Nonetheless, the stroke risk in AF patients is not homogeneous2 but depends on the presence of other underlying clinical conditions.3 These risk factors have been used to formulate stroke risk schemes that are used in clinical practice to stratify the embolic risk (low, moderate, or high) in AF and to choose proper antithrombotic agents, especially since until recently we only had an “inconvenient” anticoagulant, the vitamin K antagonist.4, 5 Oral anticoagulation (OAC) is highly effective in reducing stroke risk and mortality rates in patients with AF,6 but also raises the risk for bleeds, at least in the historical trials.7, 8 More contemporary data show that the risk of major bleeding with acetylsalicylic acid may not be significantly different from OAC, especially in the elderly.9, 10, 11.
Several risk stratification schemes have been derived from nonwarfarin arms of clinical cohort trials and/or expert consensus groups.12 The most popular risk stratification scheme has been the CHADS2 (congestive heart failure, hypertension, age, diabetes, stroke [doubled]) score13 because it is easy to remember and calculate4, 5 and in some studies may have a better predictive value than other scores.13 More recently, the value of the CHADS2 scheme has been debated, given its noninclusion of many stroke risk factors and other limitations.14, 15 Thus, the CHADS2 score has been refined with the CHA2DS2-VASc (congestive heart failure, hypertension, age≥75 [doubled], diabetes, stroke [doubled]-vascular disease and sex category [female]) emphasizing a risk factor-based approach.12 Existing risk factors have been reclassified and new risk factors have been included (such as female sex and vascular disease).12, 16 The CHA2DS2-VASc consistently outperforms the CHADS2 score in identifying low risk patients, and is as good as–and possibly better than–the CHADS2 score in identifying those who develop stroke and thromboembolism.12, 17, 18 Therefore, the European Society of Cardiology guidelines4, 5 encourage the use of CHADS2 and CHA2DS2-VASc to refine stratification of patients and to aid decisions for thromboprophylaxis. A possible criticism has suggested that this risk score cannot give us more information after initiating OAC.19 A recent Spanish study has even shown that the CHA2DS2-VASc risk stratification scheme better discriminated between patients at a low and intermediate risk of thromboembolic complications when compared to others.20.
This study aims to assess the usefulness of CHA2DS2-VASc score to differentiate and predict adverse cardiovascular outcome and mortality among patients with AF on OAC. We aimed to assess the usefulness of CHA2DS2-VASc score to give us an additional prognostic perspective for adverse events and mortality among “real world” anticoagulated patients with AF who are often elderly patients with many comorbidities..
Methods Study PopulationWe recruited 998 consecutive outpatients diagnosed as having permanent or paroxysmal nonvalvular AF from our outpatient anticoagulation clinic. All patients received acenocoumarol OAC and had stabilized international normalized ratio values (INR 2.0-3.0) for at least the 6 months before study inclusion. The CHA2DS2-VASc score was calculated as previously described.12 We selected those patients with a CHA2DS2-VASc score≥2 (high risk for stroke). For this reason, 65 (6.5%) patients were excluded. Finally 933 patients were included in the present study and followed for more than 2 years..
Inclusion criteria were an age older than 18 years, absence of any hematological disorder or contraindication for OAC in the last 6 months, absence of ischemic events (acute coronary syndrome, interventional procedures, stroke, or hemodynamic instability) requiring hospitalization at least for 6 months before a patient's enrollment, absence of rheumatic AF and prosthetic heart valves. Clinical and demographic characteristics as well as details from the antithrombotic therapies received/prescribed were recorded from their medical records (Table 1)..
Table 1. Baseline Clinical Characteristics of Atrial Fibrillation Patients on Oral Anticoagulation
| Patients | N=933 |
| Male sex | 432 (46) |
| Age, years | 76 [71-81] |
| Age≥75 years | 570 (61) |
| Hypertension | 796 (85) |
| Diabetes mellitus | 253 (27) |
| Hypercholesterolemia | 298 (32) |
| Current tobacco smoking habit | 127 (14) |
| Congestive heart failure | 360 (39) |
| Prior stroke or TIA | 190 (20) |
| Coronary artery disease | 185 (20) |
| Peripheral vascular disease | 87 (9) |
| CHA2DS2-VASc score | 4 [3-5] |
| CHADS2 score | 2 [2-3] |
| Concomitant treatment | |
| Antiplatelet therapy | 160 (17) |
| ACE inhibitors | 246 (26) |
| Angiotensin-renin blockers | 212 (23) |
| Calcium antagonist | 209 (22) |
| Beta-blockers | 285 (30) |
| Statins | 199 (21) |
| Digoxin | 177 (19) |
| Diuretics | 402 (43) |
ACE, angiotensin converting enzyme; TIA, transient ischemic attack.
Data are expressed as no. (%) or median [interquartile range].
Follow-up was performed through visits in our outpatient anticoagulation clinic. During the study period, there were no changes in the anticoagulant drug class. Dental procedures were managed without retiring OAC. We detected 60 programmed surgeries with bridging therapy with low molecular heparins without adverse events associated to them. Adverse events were recorded, including thrombotic and cardiovascular events (such as stroke both ischaemic and embolic, acute coronary syndrome, acute heart failure), major bleeding events, and global mortality and cardiovascular death. Major bleeds were determined according to the 2005 International Society on Thrombosis and Haemostasis criteria.21 Besides, “major adverse events” (MAE) were defined as a composite end-point of cardiovascular events, major bleeding, and mortality..
Statistical AnalysisVariables are presented as counts (percentages) or median [inter quartile range] as appropriate for categorical and continuous data, respectively. Kolmogorov-Smirnov test was used to check for normal distribution of continuous data. The clinical impact of the calculated CHA2DS2-VASc was determined using Cox regression modeling with the score as the dependent variable. For all the investigated adverse events (cardiovascular events, major bleeding, mortality, and composite end-point) the percentage of event-rates per year after stratification of patients from 2 to 9 points (according to the CHA2DS2-VASc scoring system) were calculated, with hazard ratio (HR) obtained for 1 point of each increase in risk scoring from Cox regression modeling. The accuracy of prognostic value from CHA2DS2-VASc score was determined by calculating the area under the receiver-operator characteristic curve and the c-statistic value. The c-statistic quantifies and discriminates the ability (P-value≥.5), whereas HR quantifies the increased relative risk of adverse events across scores stratus. All P-values<.05 were accepted as statistically significant. Statistical analysis was performed using SPSS 15.0 for Windows (SPSS, Inc., Chicago, Illinois, United States)..
ResultsBaseline clinical characteristics of the 933 (93.5%) patients included and assessed for CHA2DS2-VASc score≥2 and adverse events are shown in Table 1. The median age was 76 [71-81] years old, with 432 (46%) of them males. All patients assessed had CHA2DS2-VASc score≥2 and the median CHA2DS2-VASc score was 4 [3-5] and the median CHADS2 score was 2 [2-3]..
Median follow-up period was over 2.5 years (median 946 [782-1068] days). During this period, 109 patients (11.7%) presented with an adverse cardiovascular event, 80 patients (8.6%) had a major bleeding event, and 101 patients (10.8%) died; 30 (3.2%) of them died as a result of vascular death and 9 (0.9%) after a hemorrhagic event. As a composite end-point of cardiovascular events, MAE major bleeding and mortality was observed in 230 patients (24.6%) (Table 2)..
Table 2. Total Event Rates per Year
| End-points | no., % | Rate, %/year |
| Cardiovascular events | 109 (11.7) | 4.5 |
| Stroke | 38 (4.1) | 1.6 |
| ACS | 41 (4.4) | 1.7 |
| Acute HF | 31 (3.3) | 1.3 |
| Major bleeding | 80 (8.6) | 3.3 |
| Intracranial | 17 (1.8) | 0.7 |
| Global death | 101 (10.8) | 2.7 |
| Cardiovascular death | 30 (3.2) | 1.2 |
| Hemorrhagic cause | 9 (0.9) | 0.4 |
| MAE | 230 (24.6) | 9.5 |
ACS, acute coronary syndrome; HF, heart failure; MAE, major adverse events.
Size of the whole sample assessed was of 933 atrial fibrillation patients on oral anticoagulation and at high risk for stroke (CHA2DS2-VASc≥2). Median [interquartile range] follow-up period was 946 [782-1068] days.
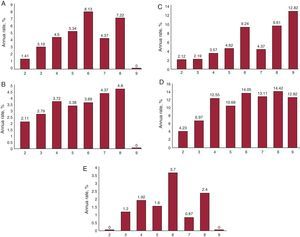
In Table 3 and Figure we present the percentage of event rates per year according to the CHA2DS2-VASc score. We clearly show increasing event rates for 1 unit-increasing CHA2DS2-VASc score for cardiovascular events (Table 3A), major bleeding episodes (Table 3B), death rate (Table 3C), and MAE (Table 3D)..
Table 3. Percentage of Event Rates per Year According the CHA2DS2-VASc Score
| CHA2DS2-VASc score | % Event rate/year, % | No | Yes | Total |
| A. Cardiovascular events | ||||
| 2 | 1.41 | 105 | 4 | 109 |
| 3 | 3.19 | 177 | 16 | 193 |
| 4 | 4.50 | 219 | 29 | 248 |
| 5 | 5.34 | 186 | 30 | 216 |
| 6 | 8.13 | 82 | 22 | 104 |
| 7 | 4.37 | 39 | 5 | 44 |
| 8 | 7.22 | 13 | 3 | 16 |
| 9 | 0 | 3 | 0 | 3 |
| Total | 824 | 109 | 933 | |
| B. Major bleeding rate | ||||
| 2 | 2.11 | 103 | 6 | 109 |
| 3 | 2.79 | 179 | 14 | 193 |
| 4 | 3.72 | 224 | 24 | 248 |
| 5 | 3.38 | 197 | 19 | 216 |
| 6 | 3.69 | 94 | 10 | 104 |
| 7 | 4.37 | 39 | 5 | 44 |
| 8 | 4.80 | 14 | 2 | 16 |
| 9 | 0 | 3 | 0 | 3 |
| Total | 853 | 80 | 933 | |
| C. Death rate | ||||
| 2 | 2.12 | 103 | 6 | 109 |
| 3 | 2.19 | 182 | 11 | 193 |
| 4 | 3.57 | 225 | 23 | 248 |
| 5 | 4.62 | 190 | 26 | 216 |
| 6 | 9.24 | 79 | 25 | 104 |
| 7 | 4.37 | 39 | 5 | 44 |
| 8 | 9.61 | 12 | 4 | 16 |
| 9 | 12.82 | 2 | 1 | 3 |
| Total | 832 | 101 | 933 | |
| D. Major adverse event rate | ||||
| 2 | 4.23 | 97 | 12 | 109 |
| 3 | 6.97 | 158 | 35 | 193 |
| 4 | 12.55 | 185 | 63 | 248 |
| 5 | 10.68 | 156 | 60 | 216 |
| 6 | 14.05 | 66 | 38 | 104 |
| 7 | 13.11 | 29 | 15 | 44 |
| 8 | 14.42 | 10 | 6 | 16 |
| 9 | 12.82 | 2 | 1 | 3 |
| Total | 703 | 230 | 933 | |
| E. Stroke rate | ||||
| 2 | 0 | 109 | 0 | 109 |
| 3 | 1.20 | 187 | 6 | 193 |
| 4 | 1.92 | 237 | 11 | 248 |
| 5 | 1.60 | 207 | 9 | 216 |
| 6 | 3.70 | 94 | 10 | 104 |
| 7 | 0.87 | 43 | 1 | 44 |
| 8 | 2.40 | 15 | 1 | 16 |
| 9 | 0 | 3 | 0 | 3 |
| Total | 895 | 38 | 985 | |
Figure. A: cardiovascular events according to CHA2DS2-VASc (annual rate). B: haemorrhagic events according to CHA2DS2-VASc score (annual rate). C: mortality according to CHA2DS2-VASc score (annual rate). D: major adverse events according to CHA2DS2-VASc score (annual rate). E: stroke according to CHA2DS2-VASc score (annual rate).
The CHA2DS2-VASc score had a c-statistic of 0.61 (95% confidence interval [95%CI], 0.59-0.66; P<.001) for cardiovascular events, while for mortality the c-statistic was 0.64 (95%CI, 0.58-0.70; P<.001), and for MAE, 0.61 (95%CI, 0.57-0.65; P<.001) (Table 4). The c-statistic for major bleeding episodes was not significant (0.54; 95%CI, 0.48-0.61; P=.179)..
Table 4. Predictive Value and Clinical Impact of Increasing CHA2DS2-VASc Score in End-Point Occurrence: C Statistic Indices and Hazard Ratios by Cox Regression Analysis
| CHA2DS2-VASc | ||||
| End-point | Predictive value c-statistic (95%CI) | P-value | Cox analysis HR (95CI%) | P-value |
| Cardiovascular events | 0.61 (0.59-0.66) | <.001 | 1.27 (1.13-1.44) | .001 |
| Major bleeding events | 0.54 (0.48-0.61) | .179 | 1.14 (0.98-1.32) | .092 |
| Mortality | 0.64 (0.58-0.70) | <.001 | 1.36 (1.19-1.54) | <.001 |
| MAE | 0.61 (0.57-0.65) | <.001 | 1.23 (1.13-1.34) | <.001 |
95%CI, 95% confidence interval; HR, hazard ratio; MAE, major adverse events (composite end-point including cardiovascular events, major bleeding and mortality).
Increasing CHA2DS2-VASc and CHADS2 scores mean an increase in one unit of the each risk stratification scores.
All P-values<.05 were considered significant.
The increases in the CHA2DS2-VASc score showed a significant association with the development of clinical events, with the occurrence of cardiovascular events (HR=1.27; 95%CI, 1.13-1.44; P=.001), all-cause mortality (HR=1.36; 95%CI, 1.19-1.54; P<.001) and MAE (HR=1.23; 95%CI, 1.13-1.34; P<.001), Table 4. There was no significant association between CHA2DS2-VASc score and major bleeding episodes (HR=1.14; 95%CI, 0.98-1.32; P=.092)..
DiscussionThe findings of the present study suggest that the CHA2DS2-VASc scoring system may be a useful tool to predict adverse events beyond thromboembolic risk in AF patients taking OAC. We found that one-unit-increasing CHA2DS2-VASc score12 in high risk patients–which ranges from 2 to 9 points−was significantly associated with higher event rate, in particular cardiovascular events and mortality, despite all patients included taking OAC. There also was no statistically significant association between CHA2DS2-VASc score and major bleeding events..
We found that increasing scores across the CHA2DS2-VASc scoring strata–explored by 1-unit increments−consistently increased by 1.23 and 1.36-fold the risk (HR) to suffer any of the adverse events or mortality. Thus, subtype stratification into different high-risk categories derived from the calculation of CHA2DS2-VASc score may reflect the reality of risk for those AF patients at high risk on OAC. In a cohort study of 11 245 patients, Baruch et al.22 concluded that high risk patients may be treated with more aggressive therapeutic strategies than those at moderate risk. Other authors have previously evaluated the risk for stroke/TE in individual AF patients according to their underlying clinical conditions,7 for example, to target their optimal INR and improve thromboprophylaxis decisions, but results were unsuccessful. This perhaps needs to be investigated in order to aid more accurate thromboprophylaxis decisions for the management of those “classical high risk patients” depending on their categorization into a “high risk subtype stratum.”.
AF patients are at high risk for both cardiovascular and bleeding events.23 Notably, a great number of risk factors included in the CHADS2 score are also bleeding risk factors,24 ie, prior stroke, elderly, renal impairment or hypertension,25 assessed by the popular HAS-BLED score.26 It means that as the risk for stroke and thromboembolism increases−measured by, for example, the CHADS2 score−the bleeding risk also increases.27, 28 With the novel OAC agents, the move has been to be more inclusive, rather than exclusive, of stroke risk factors.29 Thus, the CHA2DS2-VASc includes newer risk factors and refines point assignment to others, and in several independent cohorts, the ability of the CHA2DS2-VASc score to predict or assess the impact in the occurrence of adverse events has been compared with other current risk stratification schemes, whereby CHA2DS2-VASc consistently better identifies patients truly at low to moderate risk for stroke and thromboembolism and is as good–and possibly better−at identifying “high” risk for thromboembolism.12, 17, 30, 31, 32 We recently showed how HAS-BLED score may give important prognostic information regarding death and cardiovascular events, and not only bleeding risk.33 However, we were not able to demonstrate a significant predictive role of CHA2DS2-VASc score regarding bleeding risk in the present cohort. The median HAS-BLED score in our population was 2 [2-3]. It may explain, at least in part, the lower bleeding risk in our population. We have recently demonstrated in a population on acenocoumarol OAC that bleeding rates only exceeded thrombotic events at HAS-BLED score≥333 as previously demonstrated.27 Moreover, acenocoumarol, given its pharmacokinetic features34 which may increase the risk of having INR>6, must be the better recommendation for patients at “low hemorrhage risk” to achieve OAC into during time in therapeutic range (TTR). Noteworthy, although a lesser number of studies have compared therapeutic effects of acenocoumarol vs warfarin, acenocoumarol appears to lead to less stable TTR,34, 35 a disadvantageous effect of acenocoumarol therapy which is not found in our selected population. Thereby, the relative low bleeding risk and acenocoumarol based-on anticoagulation, together with the higher TTR at entry of our cohort, may result in a more stable population with reduced thrombotic and hemorrhagic risk. It may explain the modest predictive value of CHA2DS2-VASc reported in our study. Accordingly, future investigational research should explore the clinical impact and predictive value of CHA2DS2-VASc score in those patients at 60% to 65% TTR on acenocoumarol-based anticoagulation (as warfarin-based populations finding consistent predictive value for thrombotic and hemorrhagic events after assessing the CHADS-VASc score) and/or HAS-BLED score≥3..
Most of the current risk stratification schemes are derived from nonwarfarin arms of historical clinical trial cohorts (which randomized <10% of subjects screened), in which the risk factors are often inadequately defined or recorded. Moreover, their predictive ability in patients receiving OAC is lesser validated.36 A few validation studies—some recent meta-analyses−have not been based on clinical trial cohorts, have applied the published schemes to unselected patients encountered in general clinical practice to compare their predictive value12, 17, 31, 32 and in some cases their published results were performed in selected patients without indication for OAC,30 unlike our study..
LimitationsWe included only patients under steady oral OAC to homogenize the cohort, and so other potential variables were excluded. We have recruited a population with good anticoagulation control at entry, while other clinical cohort studies recruited only patients with TTRs of 60% to 75%. Therefore, our results may not be applicable to unstable anticoagulation patients (with low TTRs) who are more prone to suffer adverse events or to patients under early OAC who are more likely to have thrombotic events27, 37. Our patients were only anticoagulated with acenocoumarol (the vitamin K antagonist most widely used in Spain) which differs from warfarin in its shorter half-life; that seems to have some advantages in clinical practice. We have found a modest predictive value of the CHA2DS2-VASc score (<70%), unlike available data from previous reports. The good OAC at entry, the use of acenocoumarol, and thus the more stable population assessed in our study might explain the modest c-statistic reported. Moreover, the exponential increase in stroke rate, previously reported,13 is blunted at higher scores probably due to the reduced number of patients in our study having high risk of stroke. It may be a limitation to achieving statistical differences. CHA2DS2-VASc is a refinement of the CHADS2 score and offers consistently better discrimination of patients at low and moderate risk,18 and is as good—and possibly better—at identifying patients at high risk of developing thromboembolic events. Hence, the exponential increasing stroke risk by CHADS2 may be labile when assessed by CHA2DS2-VASc due to the more intense stratification risk into a higher number of high risk categories. We have assessed Caucasian-based populations without any prevalence of other races, thus our results might be specific to our patient population and the way they were managed..
ConclusionsIn conclusion, the CHA2DS2-VASc score successfully predicts cardiovascular events and mortality, but not major bleeds, among high risk AF patients on OAC..
Conflicts of interestGregory Y.H. Lip has served as a consultant for Bayer, Astellas, Merck, AstraZeneca, Sanofi, BMS/Pfizer, Biotronik, Portola, and Boehringer Ingelheim and has been on the speakers bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, and Sanofi-Aventis. Francisco Marín has served as a consultant for Bayer and Boehringer Ingelheim, and has been on the speakers bureau for Boehringer Ingelheim and Boston Scientific..
Acknowledgements
This work was partially supported by Sociedad Española de Cardiología and by RD06/0014/039 (RECAVA) and PI11/1256 from Instituto de Salud Carlos III (ISCIII). E. Jover holds a research grant from ISCIII. Dr. Hernández-Romero holds a postdoctoral position funded by the Instituto de Salud Carlos III..
Received 12 December 2011
Accepted 5 February 2012
Corresponding author. Servicio de Cardiología, Hospital Universitario Virgen de la Arrixaca, Ctra. Madrid-Cartagena s/n, 30120 El Palmar, Murcia, Spain. fcomarino@hotmail.com