Percutaneous techniques for the treatment of mitral regurgitation have aroused much interest in recent years. Percutaneous mitral annuloplasty can be performed indirectly by using devices implanted in the coronary sinus or directly by using a retrograde approach. However, as yet, the results of these techniques are scarce and some devices have a high complications rate. The most frequent percutaneous mitral valve repair technique consists of mitral leaflet plication by implanting 1 or more percutaneous clips (MitraClip) in an imitation of the Alfieri surgical technique. Clinical experience with this device is broader than that with any other. The MitraClip device is associated with improved mitral regurgitation in a high percentage of carefully-selected patients. However, the single randomized study performed to date (EVEREST) showed its efficacy to be less than that of surgical repair and we await the results of new randomized studies that should clarify which patient-type can benefit most from this technique. Other left ventricular remodeling devices, tendinous cord implantation, and leaflet ablation are currently undergoing preclinical development or first-in-human experimentation. Finally, the development of biological prostheses for percutaneous mitral valve replacement is at an early stage. Many promising experiments at the preclinical phase and initial experiments in humans will very probably multiply in the near future. However, the true role of this technique in treating mitral valve disease will have to be evaluated in appropriately designed randomized controlled studies.
Keywords
Mitral regurgitation (MR) is the most common valvular heart disease in the United States and the second most frequent condition requiring surgery in Europe.1 The prevalence increases with age and typically affects patients aged 65 years or older. Hence, the with the progressive aging of the population, the prevalence of MR is expected to increase in the coming years.2 The mitral valve (MV) apparatus is an anatomically complex structure3 with several components (mitral leaflets, papillary muscles, tendinous cords and annulus) which, together with the left ventricle (LV) and left atrium, influence valve hemodynamics. Any alteration to one of these can favor the development of MR. MR mechanisms can be classified in 2 broad-ranging groups as a function of the underlying disorder: organic or primary when MR originates in an intrinsic alteration of the MV, and functional or secondary, when the MV is structurally normal and the origin of the MR is a dysfunction of the LV.1 Surgery is the treatment of choice in severe chronic and symptomatic or asymptomatic MR with ventricular dysfunction, recent-onset atrial fibrillation or pulmonary hypertension.4,5 In fact, up to 33% of patients (62% in moderate-to-severe MR) have had some type of cardiac event at 5 years despite medical treatment6 and very few with severe MR survive long-term without intervention.7 Despite the lack of randomized studies, MV repair surgery, when feasible, is preferable to valve replacement as it is associated with lower rates of short- and long-term mortality and morbidity, better preservation of ventricular function, and the chance of avoiding anticoagulant therapy.5 However, up to 49% of patients with severe MR are contraindicated for surgery because of their age or because they have ventricular dysfunction or other comorbidities8; among patients indicated for surgery, only 34% to 53% undergo MV repair.9 Moreover, the benefit of surgery in functional or ischemic MR–which is increasingly prevalent–remains controversial.5 In recent years, percutaneous treatment of MR has aroused great interest and several devices have been developed for percutaneous MV repair and replacement (Fig. 1). In this article, we present the principle characteristics of devices and procedures and the major clinical results associated with devices intended for percutaneous treatment of MV disorders. To this end, we reviewed the literature using PubMed, EMBASE, the Cochrane Library and online sources,10–12 from October 2003 to December 2012, with the following terms: “transcatheter/percutaneous mitral valve repair, transcatheter/percutaneous mitral valve replacement, transcatheter/percutaneous mitral annuloplasty, transcatheter/percutaneous and mitral regurgitation”.
Classification of percutaneous therapies for the treatment of mitral regurgitation. CS, coronary sinus; MT, mini-thoracotomy; TA, transapical; TF, transfermoral; TP, transpericardial; TS, transseptal. aDevices with European Union approval for clinical use. bDevices with first-in-human experiments. cDevices at the preclinical stage.
Percutaneous MV repair is based on the same principles as MV surgery (partial resectioning of the leaflets, leaflet plication, annuloplasty, papillary modification and LV remodeling). For each surgical technique, there is a percutaneous equivalent.
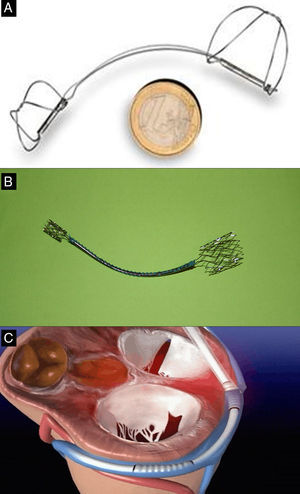
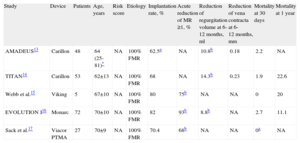
AnnuloplastyIndirect Annuloplasty via the Coronary SinusThis technique aims to imitate the effect of the ring in surgical annuloplasty by inserting a shortening device into the coronary sinus (CS) and exploits the anatomical proximity of the CS to the MV annulus. Three devices have been used in humans: the Carillon system (Cardiac Dimension; Kirkland, Washington, United States); Monarc, formerly Viking (Edwards Lifesciences; Irvine, California, United States); and Viacor PTMA (Viacor; Wilmington, Massachusetts, United States) (Fig. 2). Table 1 summarizes the major results with these devices.
Indirect percutaneous annuloplasty devices A: Carillon. B: Monarc. C: Viacor. Courtesy of Drs. David Reuter (Seattle Children's Hospital, Seattle, United States) (A), Jan Harnek (Skane University Hospital, Sweden) (B) and Stefan Sack (Academic General Hospital, Munich, Germany) (C).
Results With Direct Annuloplasty Devices
| Study | Device | Patients | Age, years | Risk score | Etiology | Implantation rate, % | Acute reduction of MR ≥1, % | Reduction of regurgitation volume at 6-12 months, ml | Reduction of vena contracta at 6-12 months, mm | Mortality at 30 days | Mortality at 1 year |
| AMADEUS13 | Carillon | 48 | 64 (25-81)* | NA | 100% FMR | 62.5a | NA | 10.8b | 0.18 | 2.2 | NA |
| TITAN14 | Carillon | 53 | 62±13 | NA | 100% FMR | 68 | NA | 14.3b | 0.23 | 1.9 | 22.6 |
| Webb et al.15 | Viking | 5 | 67±10 | NA | 100% FMR | 80 | 75b | NA | NA | 0 | 20 |
| EVOLUTION I16 | Monarc | 72 | 70±10 | NA | 100% FMR | 82 | 93b | 8.8b | NA | 2.7 | 11.1 |
| Sack et al.17 | Viacor PTMA | 27 | 70±9 | NA | 100% FMR | 70.4 | 68b | NA | NA | 0a | NA |
FMR, functional mitral regurgitation; MR, mitral regurgitation; NA, not available.
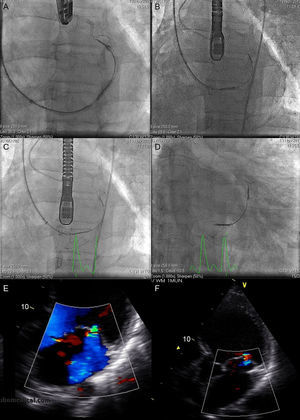
The Carillon system (Fig. 2A) is self-expandable with distal and proximal anchors connected by a nitinol cable. These are placed and released in the great cardiac vein and proximal CS, respectively, via puncture of the jugular vein and a specially-designed delivery catheter (9F). Once the distal anchor has been implanted, manual traction is applied to the device release system to regulate the degree of annular contraction (Fig. 3). The feasibility, safety and efficacy of this device in patients with functional MR secondary to dilated cardiomyopathy was determined in AMADEUS study.13 Delivery was impossible in 18 of 48 patients (37.5%) due to access-related difficulties (n=5), the impossibility of releasing the device in an adequate position (n=3), insufficient MR reduction (n=4) or coronary artery compromise (n=6). In patients with successful implantation, improvement was observed in ventricular dimensions and functional capacity at 6 months. The periprocedural complication rate was 13% (mortality, 2.2%; myocardial infarction, 6.5%; CS perforation/dissection, 6.5%). Recently, the results of the TITAN study have been published: the Carillon device was implanted in 53 patients and had to be withdrawn following implantation in 17 (32%) due to coronary obstruction (n=8) or absence of MR reduction (n=9).14 A significant reduction of regurgitation volume and LV dimensions was found in patients with definitive implantation, and clinical improvement persisted at the 2-year follow-up. During follow-up, fractures of the anchor wire were recorded in 25% of patients. Preliminary data from the ongoing TITAN II clinical trial18 highlight a higher successful implantation rate (∼ 80%) and a fall in the incidence of device fracture in comparison with TITAN. To date, Carrillon is the only percutaneous annuloplasty device with European Union approval and the PRIME study, representing initial post-commercialization experience of this device, is currently under way.
Fluoroscopy sequence of Carillon device implantation and echocardiographic findings A: distal anchor implantation. B: applying tension to the system with change in annulus size and catheter configuration. C: proximal anchor implantation to ensure tension in the system. D: released device. E: mitral regurgitation before the procedure. F: mitral regurgitation after the procedure. Courtesy of Dr. David Reuter (Seattle Children's Hospital, Seattle, United States).
The Monarc device is implanted in the CS in a similar way via a catheter guidewire to facilitate positioning. This device has 3 sections (Fig. 2B): self-expanding proximal and distal anchors with a spring-like bridge between them, partly covered with biodegradable material that is absorbed, shortening the device as it disappears over some 1 to 2 months. The distal anchor is deployed in the anterior interventricular vein and the proximal anchor in the CS ostium, and the device covers a greater proportion of the mitral annulus circumference than other percutaneous annuloplasty systems. The acute effect of the device is determined during the procedure and it can be withdrawn if the result is not optimal. However, once it has been released, the result should be determined after reabsorption of the bridge material—a process that takes place after an interval of several weeks. No method of predicting the subacute response of the device exists. Initial experience with the first version of the device (Viking) showed significant MR reduction (1-2 grades) but a high percentage (60%) of fractures in the area of the bridge.15 In a more recent version of the device (Monarc), the bridge has been reinforced to avoid fractures. Monarc was used in patients with (ischemic or idiopathic) dilated cardiomyopathy and 2+ or more MR in the EVOLUTION I16 Implantation was successful in 82% (in 13 of 79 patients implantation was impossible mainly because of difficulties due to anatomic variations of the CS) and in 50% an improvement of at least 1 grade of MR was found at the 1-year follow-up. Some 30% of patients (15 out of 50) with angiographic follow-up had a coronary compression complication (circumflex artery or anterior descending artery diagonal branch in all cases), further complicated by clinical infarction in 2 patients (4%). No fractures in the area of the bridge were found but clinically inconspicuous fractures in the anchor section proximal to the bridge were observed in 8% of the patients. After EVOLUTION II was suspended due to the low enrollment rate, production of the device ceased.
The Viacor PTMA device uses a different mechanism, without the need to encompass and contract the entire mitral annulus. Using a 3-lumen release system (7 Fr) deployed in the CS, up to 3 nitinol rods of different lengths and stiffness are simultaneously positioned in the central and posterior areas of the mitral annulus, so as to reduce the septal-lateral dimension (Fig. 2C). During the procedure, more rods can be added until the required degree of compression and MR reduction has been achieved. One potential advantage of this device is that the rods can be replaced by others that are more rigid or can be recovered in a second procedure should any complication arise (eg, coronary obstruction) or if they fail to achieve efficacy. Implantation in humans is feasible but the success rate is less than 50% (33%-45%).17,19
Several limitations are common to all 3 devices. The mechanical stress of the CS probably exceeds estimates and device fracture due to metal fatigue has often been reported. Studies including a greater number of patients and longer follow-up are needed to determine whether the torsion generated in the CS continues to be a long-term problem for device integrity. A second limitation is extrinsic compression of a coronary artery. The CS crosses some diagonal branches in 16% of patients, or the circumflex artery and/or its marginal branches in 60%-80%.20–22 With the Carillon device, compression can be determined during the procedure and tension can be adjusted accordingly; the device can even be withdrawn. However, with the Monarc device, compression forces cannot be determined until some weeks have after complete shortening of the device is complete. Although the Viacor PTMA does not encompass the entire mitral circumference, coronary compression has also been described.23 Finally, interindividual variability in the distance between the CS and the mitral annulus is substantial, and is greater still in patients with severe MR due to dilatation of the annulus,20 which reduces device efficacy. Despite these limitations, indirect annuloplasty via the CS remains an interesting approach in the percutaneous treatment of MR, especially because of the simplicity of this approach due to easy access to the mitral structure.
To resolve some of these limitations, percutaneous mitral cerclage annuloplasty has been developed, although at the time of writing, it has only been tested in animal models. The technique involves using a coronary angioplasty guidewire (0.014”) to create a loop around the mitral annulus–later replaced by a suture; a rigid-arch nitinol device is also implanted in the CS to avoid coronary compression. Initially the guidewire is introduced through the CS, great cardiac vein, and first septal perforator to reach the right chambers, where it is ensnared and later replaced by a suture and a tension-fixation device. The suture constrains the mitral annulus and LV outflow tract. In an animal model of ischemic MR, the device was successfully implanted in 88% of the animals; acute shortening of the septal-lateral distance was achieved without modifying outflow tract dimensions, and MR and ventricular volume were reduced.24 Nonetheless, although this device potentially overcomes the limitations due to anatomic variations of the CS and coronary compression, with initially promising results, its feasibility, safety and efficacy in humans need to be proven.
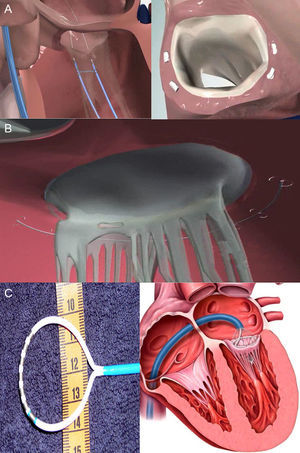
Direct AnnuloplastyThis technique also aims to avoid the limitations of indirect annuloplasty, using direct access to the mitral annulus and following the same principle of the ring as in surgical annuloplasty. However, direct access is technically more complex than access through the CS. Through a retrograde approach via the femoral artery, the catheter is positioned in the LV below the posterior mitral leaflet to access the mitral annulus. The results of the first trials in humans are available for 2 devices: Mitralign (Mitralign; Tewksbury, Massachusetts, United States) and Accucinch GDS (Guided Delivery Systems; Santa Clara, California, United States).
The Mitralign system has 2 anchors that are directly positioned in the posterior part of the mitral annulus and connected by a suture permitting adjustment of the distance between the anchors (Fig. 4A), facilitating 1cm to 3cm reductions in MV circumference.25 The current, prospective ALIGN (Mitralign Percutaneous Annuloplasty System for Chronic Functional Mitral Valve Regurgitation) clinical trial (ClinicalTrials.gov: NCT01740583) aims to evaluate the feasibility and safety of Mitralign in 50 patients with MR grade 2+ or greater and ventricular dysfunction (ejection fraction, 20%-45%). The second direct annuloplasty system is the Accucinch GDS device (Fig. 4B), which uses 9 to 12 nitinol anchors around the mitral annulus connected by a suture to exercise tension over them. Recently, the first cases of a percutaneous transfemoral approach in humans have been presented.26 Finally, the QuantumCor system (QuantumCor; Lake Forest, California, United States) consists of a circular probe with thermal electrodes, which induce scarring and annular shortening following radiofrequency ablation of different points of the mitral annulus (Fig. 4C). It is advanced via a transseptal approach and has been tested in animal models.27
The advantage of direct annuloplasty is that it avoids coronary compression and can potentially achieve a greater reduction in the grade of MR. However, the technique is much more complicated than that used in indirect annuloplasty and, at present, little is known of the mechanical effect of these devices on the MV.
Leaflet Plication or the “Edge-to-Edge” Technique: MitraClipThis technique is based on the method described by Alfieri et al.28 in 1992. It consists of suturing the edge of the anterior leaflet to the posterior leaflet, reestablishing valve coaptation and creating a double-orifice MV. The plication is usually in the central portion of each leaflet (segments A2 and P2), although stitching the edges of the commissure has also been described. The Alfieri technique is generally used in combination with annuloplasty, although the efficacy and durability of the results have also been tested in a small series with a 12-year follow-up.29
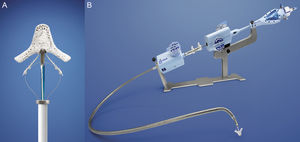
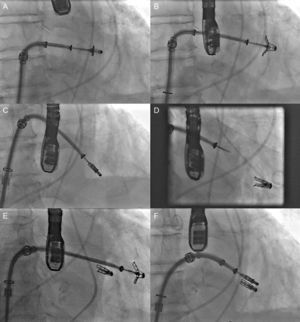
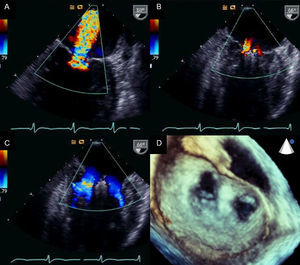
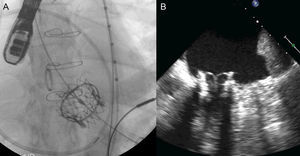
Percutaneous implantation with the MitraClip system (Abbot Vascular; Abbot Park, Illinois, United States) simulates the Alfieri technique. The MitraClip consists of a 4-mm wide chrome-cobalt clip with 2 articulated arms that open from 0° (closed position) to 240° (open position), allowing it to grasp and draw the anterior and posterior leaflets together (Fig. 5). The inner part of the arms is lined with small thorn-like teeth that guarantee adequate anchoring of the leaflets once the device has been closed (Fig. 5A). The outer part is covered in a polyester mesh to promote tissue growth and the formation of a fibrous tissue bridge between the leaflets. The MitraClip device is delivered using a 24-Fr catheter guidewire with a mobile steerable tip to position the clip (Fig. 5B). The catheter guidewire has 2 knobs that control the anterior-posterior and medial-lateral steering of the catheter tip and facilitate the opening, closure, and release of the clip. The procedure normally takes place under general anesthesia using fluoroscopy and transesophageal echocardiography (TEE), although the use of local anesthesia and sedation has been described.30,31 Femoral vein access is required with transseptal puncture to gain access to the left atrium (Fig. 6A). After reaching the mitral orifice, the clip is partially opened to pass through the MV and enter the LV. After opening the clip in the ventricular chamber, the system is withdrawn until the 2 mitral leaflets have been captured (Fig. 6B) and later closed (Fig. 6C). At this point, rigorous TEE evaluation of the location where the leaflets have been captured and of residual MR volume is vital. If the result is optimal, the clip is released (Fig. 6D) and MR is determined for a second time. If the result is suboptimal (absence of MR reduction, appearance of significant mitral stenosis), the clip can be reopened for repositioning. If the result remains suboptimal after several attempts, the clip can be fully withdrawn. If significant residual MR persists after implanting a first clip, another alternative is to implant a second clip to reduce the residual MR grade (Figs. 6E and F). As in MV repair surgery,32 TEE is essential during the procedure to determine the efficacy of the device. Figure 7 provides an example of MR before and after the procedure and a 3-dimensional TEE image of the double-orifice MV after MitraClip implantation.
Fluoroscopy sequence of MitraClip device implantation A: advanced to the mitral orifice. B: opening of the arms in the left ventricle chamber. C: withdrawal and closure of the arms to grasp the 2 mitral leaflets. D: device release. E: advancement and opening of a second device. F: implantation of a second MitraClip device. Courtesy of Dr. Ted Feldman (Evanston Hospital, Evanston, Illinois, United States).
Transesophageal echocardiography images before and after MitraClip device implantation A: mitral regurgitation before implantation. B: mitral regurgitation after implantation. C and D: 2-dimensional and 3-dimensional transesophageal echocardiography images of a double orifice valve. Courtesy of Drs. Howard Herrmann and Frank E. Silvestry (Hospital of the University of Pennsylvania, Pennsylvania, United States).
A second percutaneous mitral leaflet plication system is the Mobius device (Edwards Lifesciences, Inc.; Irvine, California, United States), which is also implanted via the transseptal approach and uses direct suturing of the leaflets. Although studies in animal models showed the feasibility of the system, development was interrupted after the first implantations in humans due to technical difficulties, suboptimal fluoroscopy images, and suture dehiscence.33,34 Finally, the MitraFlex system (TransCardiac Therapeutics; Atlanta, Georgia, United states), which is still undergoing preclinical studies, uses a clip via the transapical approach, and offers the chance of implanting an artificial tendinous cord in the same procedure.
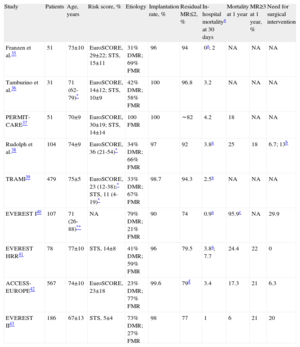
The most extensive scientific evidence on percutaneous MR treatment comes from studies of the MitraClip device, the only device tested for treatment of organic or degenerative MR. The MicraClip system has had European Union approval since 2008 and so far more than 6 000 devices have been implanted around the world. Several series35–38 and multicenter registries39–42 including a considerable number of patients and a randomized clinical trial43 support its commercialization and clinical use. The principle results of these studies are listed in Table 2.
Results With the MitraClip Device
| Study | Patients | Age, years | Risk score, % | Etiology | Implantation rate, % | Residual MR≤2, % | In-hospital mortalitya at 30 days | Mortality at 1 year | MR≥3 at 1 year, % | Need for surgical intervention |
| Franzen et al.35 | 51 | 73±10 | EuroSCORE, 29±22; STS, 15±11 | 31% DMR; 69% FMR | 96 | 94 | 0a; 2 | NA | NA | NA |
| Tamburino et al.36 | 31 | 71 (62-79)* | EuroSCORE, 14±12; STS, 10±9 | 42% DMR; 58% FMR | 100 | 96.8 | 3.2 | NA | NA | NA |
| PERMIT-CARE37 | 51 | 70±9 | EuroSCORE, 30±19; STS, 14±14 | 100 FMR | 100 | ∼82 | 4.2 | 18 | NA | NA |
| Rudolph et al.38 | 104 | 74±9 | EuroSCORE, 36 (21-54)* | 34% DMR; 66% FMR | 97 | 92 | 3.8a | 25 | 18 | 6.7; 13b |
| TRAMI39 | 479 | 75±5 | EuroSCORE, 23 (12-38);* STS, 11 (4-19)* | 33% DMR; 67% FMR | 98.7 | 94.3 | 2.5a | NA | NA | NA |
| EVEREST I40 | 107 | 71 (26-88)** | NA | 79% DMR; 21% FMR | 90 | 74 | 0.9a | 95.9c | NA | 29.9 |
| EVEREST HRR41 | 78 | 77±10 | STS, 14±8 | 41% DMR; 59% FMR | 96 | 79.5 | 3.8a; 7.7 | 24.4 | 22 | 0 |
| ACCESS-EUROPE42 | 567 | 74±10 | EuroSCORE, 23±18 | 23% DMR; 77% FMR | 99.6 | 79d | 3.4 | 17.3 | 21 | 6.3 |
| EVEREST II43 | 186 | 67±13 | STS, 5±4 | 73% DMR; 27% FMR | 98 | 77 | 1 | 6 | 21 | 20 |
DMR, degenerative or mixed mitral regurgitation; FMR, functional mitral regurgitation; MR, mitral regurgitation; NA, not available; STS, Society of Thoracic Surgeons risk score.
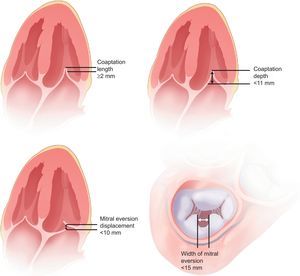
The EVEREST cohort40 is a prospective multicenter registry that analyzed the feasibility, safety and efficacy of MitraClip in patients with moderate-to-severe (3+) or severe (4+) MR with class I surgical indication. The main inclusion and exclusion criteria are summarized in Table 3. A total of 107 patients were enrolled (55 from EVEREST I and 52 in the prerandomization phase of EVEREST II), with a mean follow-up of almost 2 years. All echocardiograms were evaluated in the central echocardiography laboratory and the following anatomic criteria were established for patient selection (Fig. 8): MR jet origin in segments A2 and P2, coaptation distances of 2 mm minimum and 11 mm maximum, in the case of mitral leaflet eversion with greater than 10 mm displacement and less than 15 mm width, and more than 4 cm2 mitral area. The primary end point was residual MR of 2+ or less at 30 days following implantation of at least 1 clip. The combined efficacy end point was defined as absence of MR greater than 2+, need for surgery for valvular dysfunction, or death at 12 months. Finally, to determine device safety, adverse effects (including mortality) were prospectively recorded at 30 days and 12 months.
EVEREST Study Inclusion and Exclusion Criteria
| Inclusion criteria |
| Candidate for repair surgery or mitral valve replacement |
| Moderate-severe (grade 3) or severe (grade 4) mitral regurgitation and symptoms with LVEF>25% and LVSD≥55mm or asymptomatic with at least one of the following criteria: |
| • LVEF between 25% and 60% |
| • LVSD between 40mm and 55mm |
| • Recent-onset atrial fibrillation |
| • Pulmonary hypertension defined as pulmonary artery systolic pressure >50mmHg at rest or >60mmHg with exercise |
| Exclusion Criteria |
| Recent myocardial infarction |
| Any invasive procedure in the previous 30 days |
| Mitral valve area<4.0cm2 |
| Renal failure, endocarditis, rheumatic disease |
| Previous mediastinal surgery (in the first 27 patients) |
LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic diameter.
Anatomic criteria for MitraClip implantation in the EVEREST study. Adapted from Feldman et al.40, with permission.
Some 79% of the patients included had degenerative or functional-degenerative MR and 21% had functional MR. The clip implantation rate was 90%, and 2 clips were implanted in 29% of the patients. No device was implanted in 11 patients (10%) (due to absence of MR improvement in 8 and complications during transseptal puncture in 3). Procedural success (≤2+ MR) was 74%. In the subgroup of patients treated successfully, 77% had MR less than 2+ at discharge and 66% remained free of death, MR greater than 2+ or mitral surgery at the 1 year follow-up. Functional class and symptoms improved in 74% of patients and the results were similar in degenerative and functional MR. In terms of safety, no deaths occurred during the procedure and 10 patients (9.3%) had had an adverse event at 30 days: death (n=1), stroke (n=1), nonelective surgery (n=2), transfusions (n=4), need for reintervention due to device malfunction (n=1) and need for reintubation (n=1). No cases of clip embolization or significant mitral stenosis occurred but partial clip detachment occurred in 9% of the patients and was mostly treated by surgery.
This first, initial experience of the MitraClip device enabled researchers to establish that:
- •
The technique is safe, with a low periprocedural complication rate.
- •
It has acceptable efficacy in carefully selected patients and achieves significant MR reduction in more than two-thirds of patients.
- •
Surgery remains an alternative if treatment fails.
- •
Rigorous evaluation of MV anatomy is essential in patient selection and to obtain good results.
The EVEREST II study is a multicenter randomized clinical trial designed to compare the efficacy and safety of percutaneous treatment with MitraClip vs conventional repair surgery or MV replacement.43 Inclusion and exclusion criteria are identical to those of EVEREST I (Table 3). The echocardiographic studies were also analyzed centrally by an independent laboratory. Patients were randomized 2:1 to percutaneous therapy vs surgery. The primary efficacy end point was defined as absence of death, surgery for MV dysfunction or 3+ or 4+ MR at the 1-year follow-up. The primary safety end point at 30 days was the same as in EVEREST I.
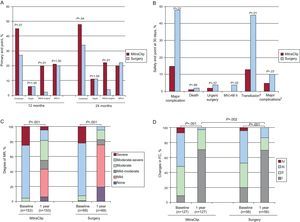
Some 258 patients with MR 3+ or 4+ (27% functional and 73% degenerative) were enrolled; 178 in the MitraClip arm and 80 in surgery. In the MitraClip group, 41 (23%) had grade 3+ or 4+ MR at discharge and 28 (16%) of them were indicated for surgery. In the surgery group, all 80 patients had MR grade 2+ or less at discharge. In the intention-to-treat analysis, 55% of the MitraClip patients met the efficacy end point at 1 year of follow-up (free of death, surgery for MV dysfunction or grade 3+ or 4+ MR), vs 73% in the surgical group (P=.007) (Fig. 9A). No differences were found in the rate of mortality or grade 3+ or 4+ MR at the 12-month follow-up. However, the MitraClip device was associated with a higher need for surgery for MV dysfunction (MitraClip, 20%; surgery, 2.2%; P<.001) (Fig. 9A). Surgical interventions were performed in 21% of the MitraClip group because the device was not implanted (n=17), grade 3+ or 4+ MR following device implantation during hospitalization (n=5), grade 3+ or 4+ MR post-implantation during follow-up (n=3), grade 3+ or 4+ MR postimplantation in a single leaflet (n=9) and symptom persistence (n=3). Differences in the primary end point vs the surgery group held at the 2-year follow-up (Fig. 9A).
Summary of the principle results of the randomized EVEREST II study A: primary end point. B: safety end point at 30 days. C: degree of mitral regurgitation. D: changes in functional class. FC, functional class; MR, mitral regurgitation; MV, mechanical ventilation. In figure B major complication includes death, infarction, stroke, surgery for mitral dysfunction, urgent surgery, infection of surgical wound,mechanical ventilation>48 h, gastrointestinal complication that requires surgery, recent-onset atrial fibrillation, septicemia and transfusion≥2 units. aTransfusion≥2 units of packed red blood cells. bExcluding transfusion.
The MitraClip device was associated with fewer major adverse events (15% vs 48%; P<.001) at 30 days, mainly due to the higher rates of transfusion in the surgery group (45% vs 13%; P<.001) and the need for intubation for more than 48 h (4% vs 0%; P=.02). No differences were found in rates of mortality, stroke, infarction, or need for urgent cardiac surgery (Fig. 9B). No device embolizations occurred. At the 1-year follow-up, the intention-to-treat analysis found a significant reduction in MR grade in both groups (P<.001), although the reduction was greater in the surgery group (Fig. 9C). Figures 9C and D summarize MR grade and functional class at the 1-year follow-up in both groups. In the subgroup analysis, the best results in the MitraClip group were obtained in patients with older age (≥70 years), functional MR, and low ejection fraction.
The EVEREST II trial showed that:
- •
Percutaneous therapy with the MitraClip device is effective in MR reduction in most patients (77%). However, although it is associated with fewer periprocedural complications than surgery (with a higher safety profile), its efficacy is clearly inferior.
- •
In most patients with no significant MR reduction with MitraClip therapy, surgery remains a feasible option.
- •
MitraClip can be an alternative therapeutic approach to surgery for selected patients with appropriate anatomic characteristics.
The EVEREST II high-risk registry (HRR) included 78 patients with moderate-severe or severe MR with an estimated surgical risk of 12% or greater (based on the Society of Thoracic Surgeons risk score or as estimated by the surgical team).41 Patient selection was based on the same echocardiographic criteria as in the randomized trial and 36 patients who failed to meet these criteria were used as a control group. Some 96% of patients received at least 1 MitraClip device; 86% of the control group underwent conservative management and 14% underwent surgery.
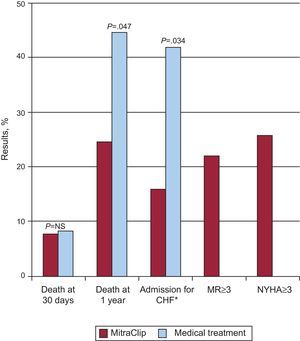
Mortality at 30 days was 7.7% in the percutaneous treatment group vs 8.3% in the control group (nonsignificant differences) (Fig. 10). At the 1-year follow-up, survival was greater in the intervention group (76.4% vs 55.3%; P=.047), with a 45% reduction in the rate of hospitalization for heart failure (P=.034). With the MitraClip device, functional class, quality of life and ventricular dimensions at 30 days and 1 year improved both in patients with functional MR and in those with degenerative MR. This study continues to enroll patients as part of the REALISM registry (clinicaltrials.gov:NCT00209274), which has 2 arms: 1 with high-risk patients (currently being recruited) and the other with nonhigh risk patients, currently with more than 650 enrollments.
European Registry: ACCESS-EUROPEThe first phase of the ACCESS EUROPE registry enrolled 567 patients with significant MR from 14 centers in 4 European countries (Germany, Denmark, Italy, and Switzerland).42 No predefined inclusion or exclusion criteria were stipulated and therefore the registry represents a “real life” registry of the application of this technique. Some 86% of the patients completed the 1-year follow-up. The patients were older and had a higher risk profile than those included in the EVEREST II study, and functional MR was 77% (Table 2). MitraClip implantation was successful in 99.6% (≥2 devices in 39%) of patients. At the 12-month follow-up, MR grade 2+ or less was maintained in 79% of the patients. Mortality at 30 days was 3.4%, and the rates of stroke, acute renal insufficiency, and cardiac taponade were 0.7%, 4.8%, and 1.1%, respectively. Mortality at 1 year of follow-up was 18.2%. No device embolization occurred and partial detachment was recorded in 4.8% of the patients. The rates of surgical intervention and percutaneous reintervention in the first year were 6.3% and 3.4%, respectively. At the 1-year follow-up, functional class had improved significantly (72% with New York Heart Association classification ≤2 at 1-year follow-up), as had quality of life and the distance covered in the 6-min walk test.
Selection of Patients for MitraClip Device ImplantationClinical evidence from multicenter registries and a randomized study show that the MitraClip device can be implanted with relative safety in a population that varies in terms of surgical risk and mitral disease type.35–43 By comparison with EVEREST II, the EVEREST-HRR and ACCESS-EUROPE registries included higher-risk patients and a greater proportion of patients with functional MR. Anatomic criteria determined by TEE were rigorously defined in EVEREST (Fig. 8) but European experience has shown that the MitraClip device can be successfully implanted in patients with more complex MV anatomy. Although future studies will have to redefine the acceptable anatomic criteria for successful MitraClip implantation, the 2 major limitations are that these clips can cause mitral stenosis and their capacity to successfully grasp the 2 MV leaflets. Hence, possible MitraClip candidate selection is heavily influenced by clinical criteria (surgical risk), valvular dysfunction etiology, valve anatomy, and TEE image quality. In patients with degenerative MR, the EVEREST study anatomic criteria are important predictors of procedure success. Therefore, the MitraClip represents an alternative to surgical treatment for patients with degenerative MR, favorable anatomic criteria, and high surgical risk. In patients with suboptimal anatomy and high surgical risk, MitraClip therapy could be indicated in selected patients. In patients with severe functional MR and symptoms refractory to medical treatment, the MitraClip device can be considered the first therapeutic option for patients of advanced age and with comorbidities (high or prohibitive surgical risk). The COAPT study (clinicaltrials.gov: NCT01626079) hopes to include 420 high surgical risk patients with grade 3+ or more functional MR randomized 1:1 to MitraClip implantation vs standard medical treatment.44 The results will truly determine the efficacy of MitraClip therapy in treating severe functional MR in inoperable patients.
The extensive clinical experience of the MitraClip device is evidence of its efficacy in reducing MR in a high percentage of patients, which usually translates into significantly improved functional capacity. However, a series of potential device-related limitations should be considered:
- •
The results of the Alfieri technique without coadjuvant annuloplasty have been suboptimal, with substantial MR recurrence and long-term need for reintervention, principally in ischemic MR or substantial annular calcification. In fact, in vitro studies have shown that MitraClip implantation alone has lower efficacy than when accompanied by annuloplasty due to the negative effect of annular dilatation.45
- •
Quantifying MR in patients under the effects of general anesthesia is difficult46,47 and sometimes it is a challenge to distinguish between the effect of the anesthesia and that of the MitraClip device on the grade of residual MR-a key to determining efficacy. In fact, approximately 1 in 5 EVEREST II patients had no MR grade improvement after MitraClip implantation.
- •
The MitraClip device can generate MV stenosis although significant stenosis has not been described to date.48
- •
The anatomic criteria for patient selection have been relatively strict (especially in EVEREST) and at present their utility has only been demonstrated in central jet MR, which excludes a large number of patients with MR.
- •
Given that this technique has only recently been applied, no long-term data on durability and efficacy are yet available.
Direct ablation of the leaflets and tendinous cords of the MV apparatus is a new, recently-developed MV repair technique. It would be especially applicable in myxomatous-origin MR, in which MV prolapse occurs with elongation of the subvalvular apparatus. A radiofrequency catheter is used to apply thermal energy to the leaflets and tendinous cords and produce tissue retraction.49 In vitro studies have shown a reduction in MV size during all phases of the cardiac cycle, which translates into improved valvular coaptation. The radiofrequency catheter is advanced via the retrograde aortic approach and has a cryogenic tip that allows it to anchor and stabilize the catheter.50 In animal models, MR reduction, persisting for at least 6 weeks, has been seen in 50% of patients or more.50 However, there are currently no data on its effect in humans. The advantages of this technique are that no device implant is required and the MV structure is not modified; however, it does have limitations: a) thermal damage is irreversible and radiofrequency energy should be strictly controlled to avoid excessive retraction; b) neighboring structures–such as the myocardium–can be damaged; c) the duration of the effect is unknown (it could be temporary), and d) areas of thermal necrosis are generated, with as yet unknown mid- to long-term consequences.
Tendinous Cord ImplantationArtificial tendinous cords can be implanted either by the transseptal or transapical approaches to create a connection between the myocardium and the mitral leaflets. The length of the cords is adjusted to recover optimal coaptation and reduce MR. This technique is mainly used in degenerative MR.
Three devices are currently under development. Two are delivered by the transapical approach: Mitraflex (TransCardiac Therapeutics; Atlanta, Georgia, United States) and NeoChord (NeoChord, Inc.; Minnetonka, Minnesota, United States). The other, the Babic system, is delivered through the transapical and transeptal approach, which requires externalization of the sutures by the transseptal approach to position a pad and anchor it to the atrial face of the leaflet, using suture traction through the apical approach.51,52 The TACT study with Neochord included 30 patients with grade 3+ or higher MR and posterior leaflet prolapse, with an 87% implantation rate.53 In 65% of patients with successful procedures, MR was 2+ or less at 30 days.
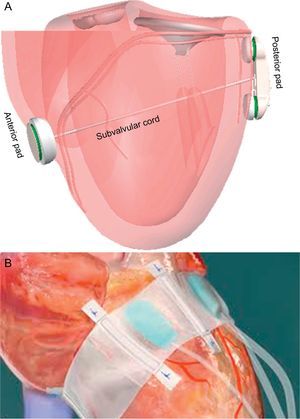
Left Ventricular RemodelingThe percutaneous iCoapsys technique is based on the Coapsys surgical system (Edwards Lifesciences; Irvine, California, United States) (Fig. 11A) and involves implanting 2 epicardial pads on both sides of the LV, joined by a flexible polyethylene cord that crosses the ventricular chamber and applies tension to the mitral annulus and LV basal wall. Indirectly, the septal-lateral distance is reduced and the papillary muscles are drawn closer to the leaflets. This is adequate for functional ischemic MR or MR secondary to cardiomyopathy. Surgical data have shown acute MR reduction and positive LV remodeling.54 Although transpericardial access via the subxiphoid approach was feasible in animals,55 in humans development has been halted.
Ventricular remodeling devices A: iCoapsys. B: Mardil-BACE. Adapted from Pedersen et al.55, with permission.
The Mardil-BACE system (Mardil, Inc.; Morrisville, North Carolina, United States) is not completely percutaneous because it requires mini-thoracotomy, although extracorporeal circulation is not needed. A silicon band is inflated around the atrioventricular notch; inflation can be adjusted after the procedure by means of a subcutaneous connection (Fig. 11B).56 This device facilitates modification of mitral annulus shape to improve leaflet coaptation. The device was implanted in 11 patients undergoing coronary bypass and a mean 2.5-grade MR reduction was achieved.57 Although clinical evidence of LV remodeling techniques for MR treatment have shown promising results, more data are needed to draw conclusions about its safety and efficacy.
PERCUTANEOUS TREATMENT OF MITRAL VALVE REPLACEMENTNative ValveTranscatheter aortic valve replacement in high surgical risk patients has progressed substantially in recent years. Equally, percutaneous MV replacement could potentially become the technique of choice in patients with severe MR and high surgical risk or those who are rejected for surgery. However, MV structural complexity, its varied etiology and our incomplete knowledge of the pathologic etiology of the MR mechanism have prevented development of percutaneous MV replacement parallel to percutaneous treatment of aortic stenosis. Several factors intrinsic to the MV apparatus hamper the development of a percutaneous MV prosthesis:
- •
The asymmetry of the mitral annulus and absence of a single valvular plane.
- •
The constant movement of the mitral annulus and the basal part of the LV hampers stable anchoring of the prosthesis.
- •
The fact that the MV is large and is close to the aortic valve and LV outflow tract.
- •
Paravalvular leaks in the mitral position are less well-tolerated than elsewhere due to the high gradients through the valves.
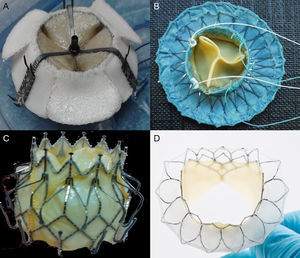
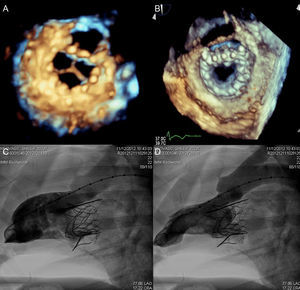
Several experimental models are under development and the first MV replacement via the transseptal approach has recently been performed in native valve in humans.58 Data are available on 4 devices currently under development (Fig. 12). The Endovalve-Herrmann prosthesis (Endovalve, Inc.; Princeton, New Jersey, United States) (Fig. 12A) is implanted by mini-thoractomy through the left atrium with no need for extracorporeal circulation. The valve is anchored using specially designed teeth and can be completely repositioned prior to release. The bovine pericardium Lutter valve (University of Kiel, Germany) is mounted on a self-expandable nitinol stent (Fig. 12B) and has been implanted in animal models via the transapical approach.59 The porcine pericardium CardiAQ valve (CardiAQ Valve Technologies, Inc.; Winchester, Massachusetts, United States) is mounted on a nitinol self-expandable stent designed for transseptal approach implantation (Fig. 12C). This is the first percutaneous valve implanted in the mitral position in a native valve.58 The procedure was performed in Denmark in an 86-year-old man with severe MR (4/4) and multiple comorbidities. Residual MR was grade 1+ after the procedure, and after an initially favorable clinical course, the patient died of multiorgan failure at day 3 postprocedure. The autopsy found no structural failure of the valve. Finally, the bovine pericardium Tiara valve (Neovasc, Inc.; Richmond, British Columbia, Canada) is mounted on a self-expandable stent, with a D-shaped atrial portion that adjusts better to the anatomy of the mitral annulus and avoids LV outflow tract obstruction (Fig. 12D). The ventricular portion has an outer coating to avoid paravalvular leaks and 3 anchor structures. It is implanted via a transapical approach with a 30-Fr catheter. The results in animals are promising, with a successful implantation rate of 81% and no significant paravalvular leaks or outflow tract obstruction (Fig. 13).60
Percutaneous mitral valves A: Endovalve-Herrmann, courtsey of Dr. Howard Herrmann (University of Pennsylvania, United States). B: Lutter valve, reproduced with permission from Drs. Lino and Lutter et al.59 C: CardiAQ valve. D: Tiara valve.
Three-dimensional echocardiography image of the Tiara valve in an animal model, with ventricular (A) and atrial (B) views and left ventricular angiography in diastole (C) and systole (D). Reproduced with permission from Banai et al.60
In recent years, in the treatment of MV disease, MV repair and the use of biological valves have increased by comparison with valve replacement using mechanical valve prostheses. However, over time, the 5% to 10% disease recurrence rates have mandated the use of new and frequently complex surgical techniques.1 In recent years, transcatheter valve replacement has become an interesting alternative for patients with previous MV surgery (biological prostheses, mitral annulus) and very high or prohibitive surgical risk.
Recently, implantation of the Melody valve (Medtronic; Minneapolis, Minnesota, United States) has been described in animal models with previous surgical annuloplasty (Melody valve in annulus) using a transseptal approach.61,62 Ten sheep underwent the procedure, using 4 different surgical annulus implants, and valve implantation was successful in all but 1 sheep.62 No paravalvular MR occurred and only 1 sheep showed central moderate-severe MR following the procedure. The pathologic study showed safe anchorage and correct sealing of the valve stent.62
Several cases of transcatheter valve implantation in patients with previous MV surgery have been reported. Although transatrial and transseptal approaches have been trialled in animal63 and human models,64–66 the transapical approach, due to its more direct access and coaxial position relative to the mitral plane, has been most extensively reported.67–70 The results have been acceptable, with minimum residual MR, although mean residual gradients are slightly high (around 6-7 mmHg). All patients have received expandable, balloon-type valves–principally Edwards-SAPIEN devices (Edwards Lifesciences Inc.; Irvine, California, United States) (Fig. 14). The percutaneous valve stent ensures adequate anchorage and sealing within the surgical annulus, avoiding paravalvular leaks. In these patients, measuring the internal diameter of the surgical prosthesis is particularly important because the manufacturer's specifications usually give the external diameter. Overdimensioning is limited by the stiff annulus, and infraexpansion of the valve could increase gradients by distorting leaflets and increasing the risk of early dysfunction. The preliminary results have recently been published of a series of 91 high-risk patients (Society of Thoracic Surgeons risk score =16.3 and logistic EuroSCORE 30%) with a median of 9 years following previous MV surgery (82 bioprostheses and 9 anulli).71 MV dysfunction was due to MR, stenosis or both in 46%, 25%, and 29% of patients. Access was predominantly via the transapical approach (86%), although some were transseptal (10%, including 1 jugular approach) and direct left atrium puncture (4%). The mean gradient and area post-procedure were 6.4 mmHg and 1.96 cm2, respectively, with 4% of patients with grade 2+ MR or higher. Mortality at 30 days was 12%, with a 1% rate of stroke and an 18% rate of major hemorrhage. Functional class improved significantly with 78% of patients in New York Heart Association class 2 or less at 30 days and annual survival of 74.5%.
Current experience with percutaneous MV replacement techniques is very scarce and surgical MV repair techniques have shown greater benefits than valve replacement, in part due to the conservation of tendinous cords and papillary muscles.4,72 This could also occur in relation with percutaneous therapies, although currently there is no evidence that MV that conserves these structures can have the same benefit as MV repair.73
CONCLUSIONSIn recent years, percutaneous treatment of MR has appeared as an alternative to MV repair/replacement surgery. Although direct and indirect annuloplasty techniques have been trialled with differing results, leaflet plication with the MitraClip device has undoubtedly been the focus of the most extensive clinical experience. Although the technique has been shown to be safer than surgery due to the low rate of periprocedural complications, its efficacy was clearly inferior to that of MV surgery. However, preliminary data indicate better survival and functional capacity with the MitraClip device than with medical treatment in patients with very high or prohibitive surgical risk. While we await the results of other randomized studies that will clarify the type of patient that benefits most from this technique, it seems obvious that the development and indications for percutaneous MR treatment should be based on patient evaluation and selection by multidisciplinary teams including interventional cardiologists, heart surgeons and echocardiographers, supported by other specialists (in geriatrics, anesthesia, etc.). Given the many MR mechanisms, use of a single percutaneous technique is unlikely to produce MR reduction similar to that following surgical repair, especially if we consider that surgery usually combines techniques oriented toward the different components involved in MR. Therefore, combining several percutaneous techniques in a single procedure or several stepwise procedures probably offers the only chance of achieving results similar to those obtained by surgery. Finally, the development of biological prostheses for percutaneous MV replacement is at an early stage and first-in-human experiments will abound in the near future. Proof of the feasibility and safety of these valves will probably be one of the greatest advances in interventional cardiology in the coming years. However, randomized studies will have to establish the true role of this new technique in treating MV disease.
FUNDINGDr. Nombela-Franco has received funding in the form of a research grant from the Fundación Alfonso Martín Escudero, Madrid (Spain).
CONFLICTS OF INTERESTNone declared.