Treatment with ferric carboxymaltose improves symptoms, functional capacity, and quality of life in patients with chronic heart failure and iron deficiency. The aim of this study was to assess the cost-effectiveness of ferric carboxymaltose treatment vs no treatment in these patients.
MethodsWe used an economic model based on the Spanish National Health System, with a time horizon of 24 weeks. Patient characteristics and ferric carboxymaltose effectiveness (quality-adjusted life years) were taken from the Ferinject® Assessment in patients with IRon deficiency and chronic Heart Failure trial. Health care resource use and unit costs were taken either from Spanish sources, or from the above mentioned trial.
ResultsIn the base case analysis, patients treated with and without ferric carboxymaltose treatment acquired 0.335 and 0.298 quality-adjusted life years, respectively, representing a gain of 0.037 quality-adjusted life years for each treated patient. The cost per patient was €824.17 and €597.59, respectively, resulting in an additional cost of €226.58 for each treated patient. The cost of gaining 1 quality adjusted life year with ferric carboxymaltose was €6123.78. Sensitivity analyses confirmed the robustness of the model. The probability of ferric carboxymaltose being cost-effective (< €30 000 per quality-adjusted life year) and dominant (more effective and lower cost than no treatment) was 93.0% and 6.6%, respectively.
ConclusionsTreatment with ferric carboxymaltose in patients with chronic heart failure and iron deficiency, with or without anemia, is cost-effective in Spain.
Keywords
Chronic heart failure (CHF) has high prevalence,1 and major morbidity and mortality2 in Spain.
Iron deficiency is common in patients with CHF, and is found in 40% to 50%3–5 of ambulatory patients. In many cases there is an absolute iron deficiency, due to occult gastrointestinal losses (related to anticoagulant and antiplatelet therapies), but often the deficiency is functional (reticuloendothelial block), related to hepcidin production and the pro-inflammatory state of CHF.6 Recent studies have shown that iron deficiency in patients with CHF is associated with higher mortality, lower functional class, lower exercise tolerance and worse quality of life, regardless of anemia status.3–5,7 These findings suggest that in the majority of patients with CHF, anemia is secondary to iron deficiency, which in turn is a comorbidity in its own right.
The FAIR-HF (Ferinject® Assessment in patients with IRon deficiency and chronic Heart Failure) trial was a randomized, double-blind, placebo-controlled clinical trial (n=459), which assessed the clinical benefits and quality of life associated with intravenous ferric carboxymaltose (FCM) treatment in patients with CHF (NYHA [New York Heart Association] functional classes II and III, with left ventricular ejection fraction ≤ 40% for class II patients and ≤ 45% for class III patients), iron deficiency, and hemoglobin level between 9.5 and 13.5g/dL.8 Ischemia was the main cause of heart failure in the FAIR-HF trial, accounting for 245 patients (80.6%) in the FCM group and 123 (79.4%) in the placebo group. The primary and secondary efficacy endpoints were change in NYHA functional class from baseline, and change in the health-related quality of life questionnaire EuroQol (European Quality of Life) of 5 dimensions index. The FAIR-HF trial showed that FCM had a better outcome than placebo in both parameters.
Not only does heart failure have a negative impact on mortality and quality of life, but it is also associated with increased health care costs.9 This financial burden, for patients and health systems alike, accounts for almost 2% of the health care budget in most developed countries.9 In the current economic situation, Spanish and other public health care system policy makers must make key decisions to reduce chronic disease costs and assess the cost-effectiveness of new treatments. For this reason, our study aimed to assess the cost-effectiveness of iron deficiency treatment with intravenous FCM in patients with CHF, using an economic model based on the Spanish National Health System.
METHODSModelWe adapted a cost-effectiveness model to the Spanish healthcare setting, to calculate the cost of gaining one effectiveness unit with FCM treatment of iron deficiency compared with no iron treatment, in patients with CHF, with and without anemia. Effectiveness is expressed as quality-adjusted life years (QALYs), ranging from 0, or even negative values (worst health possible), to 1 (best health possible) according to patient self-assessment and tools such as EuroQol.10
The model was a theoretical scheme that simulates complex health processes.10 Its decision-tree design has been previously described in an analysis performed for the United Kingdom.11 We implemented the model with TreeAge Pro 2011 Suite (TreeAge Software Inc.; Williamstown, Massachusetts, United States).
EffectivenessWe used data from the FAIR-HF trial patients to model the patients’ clinical and demographic characteristics.8,12 We obtained QALYs per patient with or without FCM from the same FAIR-HF patients, who had completed the EuroQol-5 dimension questionnaire.8,11
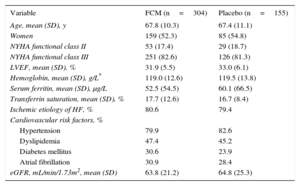
The clinical model population was made up of 459 patients, selected on an intention to treat basis (304 received FCM and 155 placebo), with iron deficiency, CHF, and the characteristics shown in Table 1. The study design, endpoints, and clinical outcomes have been described previously.8,12
Clinical and Demographic Characteristics of the Ferinject® Assessment in Patients With IRon Deficiency and Chronic Heart Failure Intention to Treat Population11
| Variable | FCM (n=304) | Placebo (n=155) |
|---|---|---|
| Age, mean (SD), y | 67.8 (10.3) | 67.4 (11.1) |
| Women | 159 (52.3) | 85 (54.8) |
| NYHA functional class II | 53 (17.4) | 29 (18.7) |
| NYHA functional class III | 251 (82.6) | 126 (81.3) |
| LVEF, mean (SD), % | 31.9 (5.5) | 33.0 (6.1) |
| Hemoglobin, mean (SD), g/L* | 119.0 (12.6) | 119.5 (13.8) |
| Serum ferritin, mean (SD), μg/L | 52.5 (54.5) | 60.1 (66.5) |
| Transferrin saturation, mean (SD), % | 17.7 (12.6) | 16.7 (8.4) |
| Ischemic etiology of HF, % | 80.6 | 79.4 |
| Cardiovascular risk factors, % | ||
| Hypertension | 79.9 | 82.6 |
| Dyslipidemia | 47.4 | 45.2 |
| Diabetes mellitus | 30.6 | 23.9 |
| Atrial fibrillation | 30.9 | 28.4 |
| eGFR, mL/min/1.73m2, mean (SD) | 63.8 (21.2) | 64.8 (25.3) |
eGFR, estimated glomerular filtration rate; FCM, ferric carboxymaltose; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
The values are expressed as mean (standard deviation), or No. (%).
We used Spanish sources and the FAIR-HF trial data for health resource use and unit costs (expressed in 2013 euros). We only considered direct health costs (National Health System cost of purchase and intravenous administration of FCM, and hospitalization for CHF). Using the FAIR-HF trial as a starting point, we estimated the mean dose of FCM, the mean number of FCM 200-mg injections, the duration and frequency of hospitalizations with and without FCM and the difference in QALYs with and without FCM8,11 (Table 2). We obtained the length of hospital stay for patients with CHF using DRG (diagnosis-related group) 127 from the DRG state standard (AP-DRG V25) by hospital group for 2010.13 Day hospital costs for patients with CHF were taken from the same source. The cost of FCM intravenous infusion in the day hospital, defined as 15min infusion time, 15min nursing time, and the cost of disposable equipment, was taken from a recent Spanish study.14 The laboratory sale price of FCM 100mg was taken from the database of the Consejo General de Colegios Oficiales de Farmacéuticos (Council of Official Colleges of Pharmacists), Bot PLUS 2.015 (Table 2).
Parameters Included in the Economic Model, Ranges and Distributions Used in the Sensitivity Analysis
| Item | Units | Value | DA variation | PA distribution |
|---|---|---|---|---|
| Mean dose of FCM administered to patients8,11 | mg | 1851.33 | 1802.12-1900.54 | Normal |
| Mean number of injections (200 mg) of FCM8,11 | — | 9.46 | 9.21-9.71 | Normal |
| Hospitalization duration in CHF13 | Days | 9.32 | 6.81-17.34 | Triangular |
| Relative hospitalization duration (FCM vs placebo)8,11 | — | 0.36 | 0.16-0.88 | Lognormal |
| Hospitalization duration with/without FCM8,11 | Days | With FCM: 1.07 | — | — |
| Without FCM: 2.95 | ||||
| Hospitalization frequency with/without FCM8,11 | — | With FCM: 0.08 | — | — |
| Without FCM: 0.17 | ||||
| Laboratory sale price of 100mg of FCM11 | Euros | 20.00 | — | — |
| Ambulatory IV FCM injection (15 min)14 | Euros | 25.11 | 18.79-32.52 | Triangular |
| Day hospital cost for CHF13 | Euros | 382.25 | 205.42-523.05 | Triangular |
| Difference in QALYs with/without FCM8,11 | — | 0.037 | 0.020-0.060 | Normal |
CHF, chronic heart failure; DA, deterministic analysis; FCM, ferric carboxymaltose; IV, intravenous; PA, probabilistic analysis; QALY, quality-adjusted life years.
Ferric carboxymaltose administration cost was calculated assuming that there would be no vial wastage, as in the FAIR-HF trial. The hospitalization cost for CHF was calculated by multiplying the number of days of hospital stay for patients with CHF by the mean number of hospitalizations in the FAIR-HF placebo group. In the case of patients treated with FCM, the number of days of hospital stay was multiplied by the mean length of stay in the FCM group divided by the mean length of stay in the placebo group. Adverse event costs were not included because no clinically relevant differences were found between the treatment groups in the FAIR-HF trial.11
Time HorizonThe time horizon of the analysis (duration of simulated patient follow-up in the model) was 24 weeks, corresponding to the duration of the FAIR-HF trial. Extrapolation of the time horizon to a longer term (eg, lifelong time horizon) was not considered adequate as the FAIR-HF trial provides no information on longer term survival or other long-term effects.11
Base Case, Sensitivity Analysis and Presentation of ResultsWe performed a baseline analysis, using mean model values for all considered variables (resource use, unit costs, and QALYs). The aim of our sensitivity analyses was to assess the stability of the baseline analysis result when considering variable outliers. Specifically, we performed univariate deterministic sensitivity analyses, and in each analysis we modified the baseline value of a determined variable, including one of the outliers for that variable, as shown in ranges in Table 2. We also carried out a probabilistic sensitivity analysis using a second-order Monte Carlo simulation, based on 10 000 sets of randomly drawn variable values, according to the statistical distributions in Table 2.11 The results are presented for one patient (with or without FCM treatment) as cost per patient, cost difference, QALYs per patient, QALYs gained with FCM, and, finally, cost per QALY gained with FCM compared with no treatment, which is the ICER (incremental cost-effectiveness ratio). Costs and benefits were not discounted given the short time horizon of the study.11
RESULTSBase CaseIn the base case analysis, each patient acquired 0.335 QALYs and 0.298 QALYs with and without FCM, respectively, with a difference of 0.037 QALYs gained in the treated patients. The cost per patient was €824.17 and €597.59, respectively, representing an additional cost of €226.58 per patient with FCM. Thus, the cost per QALY gained with FCM was €6123.78 (Table 3).
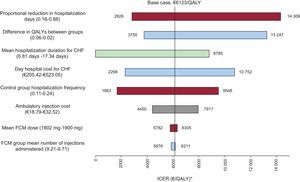
Sensitivity AnalysisThe sensitivity analyses confirmed the robustness of the base case result. The tornado diagram in Figure 1 shows the deterministic sensitivity analysis results. The most sensitive variable was the proportional reduction in hospital stay in FCM-treated patients compared with untreated patients. In the worst case, cost per QALY gained was €14 306. In all the analyses, the cost of gaining 1 QALY with FCM was < €30 000, which is the generally accepted upper threshold for cost-effective treatment in Spain.16
Results of the deterministic sensitivity analysis. Tornado diagram. Each bar represents the variability in the base case result using outliers (in parentheses) for each variable mentioned in the text on the left. CHF, chronic heart failure; FCM, ferric carboxymaltose; ICER, incremental cost-effectiveness ratio. QALY, quality-adjusted life year. *Cost per quality-adjusted life year gained.
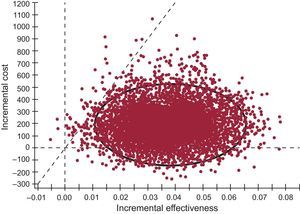
According to the Monte Carlo simulation, the probability of FCM being cost-effective (< €30 000/QALY) and dominant (more effective and lower cost than no treatment) was 93.0% and 6.6%, respectively. In terms of the ICER, 95.0% of results were between €4617 and €13 437 per QALY gained with FCM treatment compared with non-treatment (Figure 2).
Result of the probabilistic sensitivity analysis (Monte Carlo simulation). The results to the right of the diagonal broken line represent the cases in which treatment with ferric carboxymaltose was cost-effective, with cost ratios per quality-adjusted life year gained below the €30 000 threshold (which was the case in 93.0% of the 10 000 sets of analyses performed). In 6.6% of simulations, treatment with ferric carboxymaltose was dominant (more effective and lower cost than the non-treatment option). The circle encompasses the 95% confidence interval of the incremental cost-effectiveness ratio results, which range from €4617 to €13 437 per quality-adjusted life year gained with ferric carboxymaltose treatment compared with non-treatment.
This economic study shows that FCM treatment in patients with CHF and iron deficiency, with or without anemia, is cost-effective in Spain, with a cost per QALY gained of €6123.78, compared with non-treatment of iron deficiency. This is well below the €30 000 accepted threshold for cost-effective treatment in Spain.16 These results are in line with the originally published model, which reports that treatment with FCM is also cost-effective in the United Kingdom, where the acceptable threshold is €22 200 to €33 300 per QALY gained.11 The higher cost per QALY in the United Kingdom than in Spain is mainly due to the longer mean duration of hospitalization for CHF in the United Kingdom (11.8 days)11 compared with Spain (9.32 days).13
The cost-effectiveness of FCM treatment in patients with CHF and iron deficiency can be compared with other studies. One recently published European study17 on primary prophylactic defibrillator implantation in patients with ischemic or non-ischemic CHF found a cost of €43 993 per QALY gained, compared with no implantable defibrillator. Another study, in Spain, estimated that cardiac resynchronization therapy, vs drug therapy, has a cost of €28 612 per QALY gained, and that resynchronization plus defibrillator costs €53 547 per QALY vs resynchronization without defibrillator.18
LimitationsPossible limitations and consistency issues should be taken into account when assessing our study results. We used a theoretical model, which by definition is a simplified simulation of reality. However, cost-effectiveness analysis is a particularly relevant instrument which guides National Health System decision makers, because it compares the effectiveness and costs of two options and brings them together into one effectiveness variable, ie, the cost of gaining 1 QALY with the most effective comparator.19 Furthermore, the efficacy data that we used to calculate QALYs gained for FCM-treated patients come from a randomized clinical trial, which compared quality of life in patients with and without treatment as one of its objectives.8,12 In that study, patients were given repeated doses of 200mg FCM up to iron repletion (correction phase), reaching a median total dose of 1000mg in this phase. In the recently published CONFIRM trial,20,21 which had a similar design to the FAIR-HF trial, FCM was administered more rapidly according to the study protocol, with initial doses of up to 1000mg, as indicated in the summary of product characteristics. The CONFIRM trial findings confirm the FAIR-HF study results. This leads to the hypothesis that a protocol that significantly reduces the number of doses administered (from the 5 required in the correction phase of the FAIR-HF protocol, to 1 or 2 dose in the CONFIRM protocol) implies greater cost savings, and a potential lower cost per QALY gained. In the light of the CONFIRM study results, it would be useful to carry out a similar cost-effectiveness analysis to ours, with these new data.
One important limitation of our analysis is the lack of exhaustive information regarding health resource use (eg, concomitant treatments, devices such as pacemakers, and ambulatory treatments,) which was not collected in the FAIR-HF trial.8,11,12 However, there is no evidence to suggest that the use of these unmeasured resources differed between patients with and without iron treatment, and it is therefore doubtful that this information would have affected the results of the analysis.11
Sensitivity analyses endorse the reliability of cost-effectiveness results.22 In all the univariate deterministic sensitivity analyses, the cost of gaining 1 QALY with FCM was less than €30 000, which is the generally accepted upper threshold for cost-effective treatment in Spain.16 Furthermore, according to the Monte Carlo simulation, the probability of FCM being cost-effective (< €30 000 euros/QALY) and dominant (more effective and lower cost than no treatment) was 93.0% and 6.6%, respectively.
A further limitation of our analysis is that the FAIR-HF trial only assessed the effect of FCM in stable patients with left ventricular systolic dysfunction, and therefore it may not be possible to completely extrapolate these results to other patient populations with heart failure who have different stability profiles, ventricular function, or comorbidities. Nevertheless, the subgroup analysis in the original study showed that the results were consistent in all the original trial design subgroups.23
CONCLUSIONSThis study appears to show that treatment with FCM is a cost-effective option in Spain for patients with CHF and iron deficiency, with or without anemia.
FUNDINGStudy funded by Vifor Pharma España S.L.
CONFLICTS OF INTERESTNone declared.
Study performed with an unrestricted research grant from Vifor Pharma.