The recommendation for dual antiplatelet therapy following drug-eluting stent implantation ranges from 6 months to 12 months or beyond. Recent trials have suggested the safety of a 6-month dual antiplatelet therapy regimen, yet certain caveats to these studies limit the applicability of this shorter duration dual antiplatelet therapy strategy in real world settings.
MethodsA registry was constructed with consecutive recruitment of patients undergoing new-generation drug-eluting stent implantation and prescribed 6 months of dual antiplatelet therapy. Propensity score matching was undertaken with a historical cohort of patients treated with second-generation drug-eluting stents who received 12 months of dual antiplatelet therapy from the ESTROFA-2 registry. The sample size was calculated using a noninferiority basis and the primary endpoint was the combination of cardiac death, myocardial infarction, revascularization, or major bleeding at 12 months.
ResultsThe analysis included 1286 patients in each group, with no significant differences in baseline characteristics. The primary endpoint occurred in 5.0% and 6.6% in the 6-month and 12-month groups, respectively (P = .001 for noninferiority). The incidence of definite or probable stent thrombosis was 0.5% and 0.7% in the 6-month and 12-month groups, respectively (P = .4). Major bleeding events were lower in the 6-month group than in the 12-month group (0.8% vs 1.4%; P = .2)
ConclusionsIn selected patients in this large multicenter study, the safety and efficacy of a 6-month dual antiplatelet therapy regimen after implantation of new-generation drug-eluting stents appeared to be noninferior to those of a 12-month dual antiplatelet therapy regimen.
Keywords
Drug-eluting stents (DES) are associated with significantly lower rates of target lesion revascularization compared with bare-metal stents (BMS). However, long-term dual antiplatelet therapy (DAPT) is invariably required to avoid late stent thrombosis related to delayed healing, preventing the frequent use of DES in patients at high bleeding risk. For many years, guidelines have recommended a period of at least 12 months of DAPT based on the performance of first-generation DES.1 Recently, European guidelines have recommended 6 months of DAPT for stable patients (level of evidence B).2 New-generation DES trials and registries have demonstrated lower thrombosis rates compared with first-generation DES or even BMS.3–7 A retrospective analysis of new-generation DES studies reported that early discontinuation or interruption of DAPT beyond 1 to 3 months after implantation seemed not to increase the risk of risk.8,9
Several trials have compared distinct DAPT durations (3-6 months vs 12-24 months).10–16 Individual and a pooled analyses of 4 of these trials have demonstrated that shorter-term DAPT regimens show similar rates of ischemic events whilst simultaneously decreasing the incidence of bleeding events.10–17
Nevertheless, certain caveats limit the widespread applicability of these trials, including the retrospective design of analyses and associated biases, some with small sample sizes, limited adherence to protocols, and the frequent use of outdated DES. For instance, the version of the zotarolimus-eluting stent used in these trials is known to exhibit a relatively high degree of late lumen loss, earning it a reputation as being a stent whose behavior is somewhere between a BMS and the newer-generation DES. These trials also included BMS and first-generation DES.
We present a multicenter prospective registry aimed at assessing the safety of a 6-month DAPT approach in patients receiving a nonfirst-generation DES compared with the results of a matched series of patients receiving 12-months of DAPT.
METHODSThe multicenter, prospective ESTROFA-DAPT registry involves 18 centers throughout Spain. This analysis is part of the ESTROFA Project and Study Network and was supported by the Spanish Working Group of Interventional Cardiology of the Spanish Society of Cardiology. In each center patients were prescribed DAPT with acetylsalicylic acid and clopidogrel for 6 months post-DES implantation according to the following criteria:
- •
A clinical indication for percutaneous intervention with a nonfirst-generation DES in any of the following clinical settings: a) silent ischemia; b) stable angina; c) unstable angina with no degree of troponin elevation; d) patients with non–ST-segment elevation or ST-segment elevation myocardial infarction with no estimated low bleeding risk on long-term DAPT but still considered candidates for treatment with DES (ie, patients > 75 years, those with a history of peptic ulcer disease without bleeding, moderate-severe chronic renal failure, or moderate liver disease, and those with elective noncardiac surgery > 6 months).
- •
Regarding the procedure, left main coronary lesions were excluded, as well as bifurcations treated with 2 stents or patients requiring more than 3 stents. Patients with a previous history of late DES thrombosis were also excluded.
The decision to select these inclusion criteria was based on the following safety concerns: a) The use of DAPT for 12 months after an acute coronary syndrome (ACS) is supported by evidence and is recommended in various clinical guidelines and consensus documents.1,2,18–21 Only patients with low risk ACS and certain bleeding risk could be included. b) The study had a safety-driven protocol; thus, the inclusion of patients with a higher risk of stent thrombosis (> 3 stents, bifurcations with 2-stent techniques, and previous late thrombosis with DES) and those with a high risk of death under a thrombotic event (stents in left main artery) were excluded.
In fact, these subgroups (ACS, multiple lesions, and complex lesions) showed a trend to derive more benefit following a longer DAPT period vs the subgroups with stable angina or single lesions in the PRODIGY trial.10
All baseline clinical, angiographic and procedure data were reported within a common database specifically designed for this study. Information on clinical follow-up was also submitted, and these data were regularly updated during registry and hospital database reviews, as well as through patient contact. Verification of DAPT for the ≤ 6-month DAPT period was required and confirmed through patient contact. Final event adjudication was undertaken at the coordination center (Hospital Universitario Marqués de Valdecilla) by 2 blinded investigators (Drs. De la Torre and García Camarero).
To compare this cohort with the 12-month DAPT cohort, we reviewed the ESTROFA-2 study database, already published in 2010.22 This was a multicenter, prospective registry designed to assess the incidence of thrombosis following second-generation DES implantation, which included 4768 patients; among these, 4354 were treated with 12 months of DAPT. Using both registry databases (ESTROFA-DAPT and ESTROFA-2), a propensity score analysis was undertaken to obtain 2 comparable cohorts of patients treated with either a 6-month or 12-months of DAPT.
These 2 registries, although conducted in different time periods, were constructed using a similar methodology. The steering and coordination team was the same and the web-based clinical record forms shared the same format; most of the centers active in ESTROFA-DAPT also recruited patients in ESTROFA-2 (13 out of the 18 centers). Finally, the main investigators involved in analysis of the two registry databases were the same. Event adjudication was conducted with preestablished event definitions and additional information was requested as needed to achieve proper final adjudication.
Endpoints and DefinitionsThe primary endpoint of the study was 12-month event-free survival (cardiac death, myocardial infarction, revascularization and major bleeding) in both DAPT treatment groups. Secondary endpoints included all-cause death, cardiac death, nonfatal myocardial infarction, coronary revascularization, definite stent thrombosis, definite or probable stent thrombosis, definite or probable stent thrombosis in the period from 6 to 12 months after the index percutaneous coronary intervention, and major bleeding events.
The specific definitions of major adverse cardiovascular events were as follows. Myocardial infarction was defined as a typical increase and gradual fall (troponin), or as a more rapid increase and fall (creatine-kinase-MB) of biochemical markers consistent with myocardial necrosis in association with at least 1 of the following: ischemic symptoms, the development of pathological Q waves on the electrocardiogram, changes on the electrocardiogram indicating ischemia (ST-segment elevation or depression), or pathological results consistent with acute myocardial infarction. Revascularization was defined as any kind of clinically indicated percutaneous or surgical coronary revascularization. Definite or probable stent thrombosis was considered according to the definitions by the Academic Research Consortium.23 Bleeding events were categorized according to the criteria of the bleeding academic research consortium (BARC).24
Statistical AnalysisBased on previous data from ESTROFA-2 in the subgroup from this registry with a similar profile to that included in ESTROFA-DAPT, a primary endpoint rate of 6.5% to 7.0% was assumed for both groups. Therefore, with 80% power and a 1-sided type I error of 5%, a sample size of 1200 patients in each group would demonstrate noninferiority between the 2 groups for the primary end point with a noninferiority fixed margin of 2.5%, which is in accordance with noninferiority margins used in contemporary trials of DES and in a trial comparing different DAPT periods.13 If the upper bound of the 95% confidence interval of the difference in treatment (short- vs long-term DAPT) was less than 2.5%, the null hypothesis would be rejected, which would signify that the short-term group was noninferior to the long-term group with regard to the primary endpoint at 12 months.
Continuous variables are presented as mean ± standard deviation. Categorical variables are expressed as percentages. Continuous variables were compared with the Student t test if they followed a normal distribution and with Wilcoxon tests when they did not (assessment of type of distribution by the Kolmogorov-Smirnov test). The categorical variables were compared with the chi-squared test or Fischer's exact test, as required. Kaplan-Meier curves for event-free survival were obtained for each group or subgroup considered in the analysis and were compared through the log rank test. Interaction tests were conducted to identify subgroups posing different risks of stent thrombosis under the 2 different DAPT periods.
Two actions were accomplished to select comparable series of patients from these 2 registries. First, we applied the exclusion criteria from ESTROFA-DAPT to the ESTROFA-2 database, so patients with treated left main coronary lesions, bifurcations treated with 2 stents, patients with more than 3 stents implanted, and those with a previous history of late DES thrombosis were excluded from the analysis. Second, we conducted a propensity score matching process. All variables listed in Tables 1 and 2 were entered as covariates to derive the propensity scores. The “psmatching” custom dialogue was used in conjunction with SPSS version 19. The “psmatching” program performs all analyses in R though the SPSS R-Plugin (version 2.10.1). This procedure involved 3 stages: a) The propensity scores were estimated using logistic regression in which the prescription of a 6-month DAPT regimen was used as the outcome variable and all the covariates as predictors. b) Patients were matched using simple 1:1 nearest neighbor matching, which is based on a “greedy” matching algorithm that sorted the observations in the 6-month DAPT group by their estimated propensity score. This algorithm then matched each unit sequentially to a unit in the 12-month DAPT group with the closest propensity score. To exclude bad matches, we imposed a caliper of 0.2 of the standard deviation of the logit of the propensity score. We disregarded units outside the area of common support (defined as the region of the distributions of estimated propensity scores in the 6-month and 12-month DAPT groups for which units were observed in both groups). This was done to improve the balance of the covariates. c) A series of model adequacy checks was performed to check whether an adequate balance of the covariates was achieved through the matching procedure. This was done by computing the global imbalance measure and through the production of 5 diagnostic plots: histograms of the propensity scores in both groups before and after matching, a dot-plot of individual propensity scores of units in the control and treatment group, either matched or unmatched, histograms of the standardized differences of all terms (covariates, quadratic term, interactions) before and after matching, a dot-plot that displayed the magnitude of the standardized differences before and after matching for each covariate, and a line-plot of standardized mean differences before and after matching. An overall imbalance chi-squared test is provided. This test statistic, which is related to the well-known Hotelling's T2 statistic, assesses simultaneously whether any variable or any linear combination of variables is significantly unbalanced after matching. The test examined all covariates used to estimate the propensity score. Standardized differences were calculated for all covariates before and after matching to assess balance after matching. A standardized difference < 10% for a given covariate indicates a relatively low imbalance.
Clinical Characteristics in Matched Groups
| 6-month DAPT (n = 1286) | 12-month DAPT (n = 1286) | P | ASD (%) | |
|---|---|---|---|---|
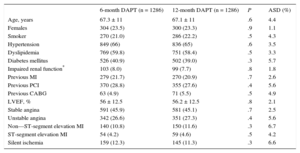
| Age, years | 67.3 ± 11 | 67.1 ± 11 | .6 | 4.4 |
| Females | 304 (23.5) | 300 (23.3) | .9 | 1.1 |
| Smoker | 270 (21.0) | 286 (22.2) | .5 | 4.3 |
| Hypertension | 849 (66) | 836 (65) | .6 | 3.5 |
| Dyslipidemia | 769 (59.8) | 751 (58.4) | .5 | 3.3 |
| Diabetes mellitus | 526 (40.9) | 502 (39.0) | .3 | 5.7 |
| Impaired renal function* | 103 (8.0) | 99 (7.7) | .8 | 1.8 |
| Previous MI | 279 (21.7) | 270 (20.9) | .7 | 2.6 |
| Previous PCI | 370 (28.8) | 355 (27.6) | .4 | 5.6 |
| Previous CABG | 63 (4.9) | 71 (5.5) | .5 | 4.9 |
| LVEF, % | 56 ± 12.5 | 56.2 ± 12.5 | .8 | 2.1 |
| Stable angina | 591 (45.9) | 581 (45.1) | .7 | 2.5 |
| Unstable angina | 342 (26.6) | 351 (27.3) | .4 | 5.6 |
| Non—ST-segment elevation MI | 140 (10.8) | 150 (11.6) | .3 | 6.7 |
| ST-segment elevation MI | 54 (4.2) | 59 (4.6) | .5 | 4.2 |
| Silent ischemia | 159 (12.3) | 145 (11.3) | .3 | 6.6 |
ASD, absolute standardized difference; CABG, coronary artery bypass graft; DAPT, dual antiplatelet therapy; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Unless otherwise indicated, data are expressed as No. (%) or mean ± standard deviation.
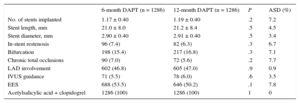
Procedural Characteristics in Matched Groups
| 6-month DAPT (n = 1286) | 12-month DAPT (n = 1286) | P | ASD (%) | |
|---|---|---|---|---|
| No. of stents implanted | 1.17 ± 0.40 | 1.19 ± 0.40 | .2 | 7.2 |
| Stent length, mm | 21.0 ± 8.0 | 21.2 ± 8.4 | .5 | 4.5 |
| Stent diameter, mm | 2.90 ± 0.40 | 2.91 ± 0.40 | .5 | 3.4 |
| In-stent restenosis | 96 (7.4) | 82 (6.3) | .3 | 6.7 |
| Bifurcation | 198 (15.4) | 217 (16.8) | .3 | 7.1 |
| Chronic total occlusions | 90 (7.0) | 72 (5.6) | .2 | 7.7 |
| LAD involvement | 602 (46.8) | 605 (47.0) | .9 | 0.9 |
| IVUS guidance | 71 (5.5) | 78 (6.0) | .6 | 3.5 |
| EES | 688 (53.5) | 646 (50.2) | .1 | 7.8 |
| Acetylsalicylic acid + clopidogrel | 1286 (100) | 1286 (100) | 1 | 0 |
ASD, absolute standardized difference; DAPT, dual antiplatelet therapy; EES, everolimus-eluting stent; IVUS, intravascular ultrasound; LAD, left anterior descending artery.
Unless otherwise indicated, DATS are expressed as No. (%), or mean ± standard deviation.
A P value <.05 was considered statistically significant. All statistical analyses were performed using SPSS version 19 for windows
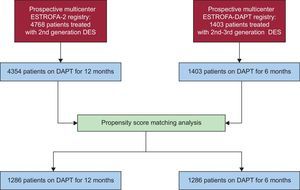
RESULTSAs shown in the study flowchart (Figure 1), from the original ESTROFA-2 and ESTROFA-DAPT cohorts and after performing propensity score matching, we obtained 2 groups of 1268 patients each: the 6-month DAPT and 12-month DAPT groups. The clinical and procedural characteristics of the 2 groups are presented in Tables 1 and 2. The estimated postmatching standardized differences for all covariates are provided. All were < 10%, which indicates an adequate balance between the groups. No differences were found in either the cardiovascular risk profile, the type of clinical presentation, or procedural characteristics between the 6-month and 12-month DAPT groups. Of note, as a result of the ESTROFA-DAPT inclusion criteria, 60% of patients in each group were in a stable clinical condition and only 15% of them had had a myocardial infarction.
Regarding DES distribution, the most frequently used DES in both groups was the everolimus-eluting stent (53.5% in the 6 month DAPT group vs 50.2% in 12 month DAPT group; P = .1). The zotarolimus-eluting stent was used in 28.8% of the 6-month DAPT group vs 49.8% in the 12-month group; coinciding with the Resolute® and Endeavor® brands, respectively. The biolimus-eluting stent was used in 12.7% of the 6-month DAPT group.
No patients were lost to follow-up and treatment adherence rates were 97% and 95% in the 6-month and 12-month DAPT groups, respectively. This means that only 3% in the 6-month DAPT group extended dual therapy beyond 6 months and only 5% in the 12-month DAPT group prolonged dual therapy beyond the first year.
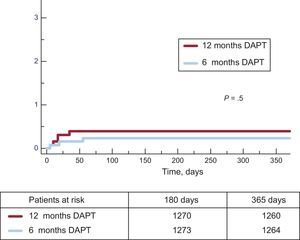
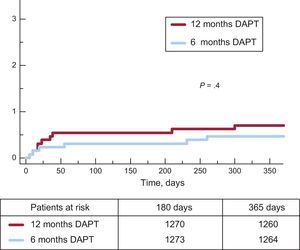
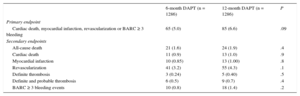
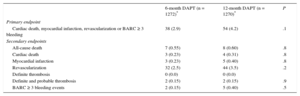
Clinical events at 12 months’ follow-up are shown in Table 3. No significant differences were observed between groups for the primary endpoint (hazard ratio = 0.75; 95% confidence interval, 0.54-1.05 for 6-month vs 12-month DAPT) or any of the endpoints considered, yielding a P = .001 for noninferiority. Not only was there a similar incidence of ischemic events in the 6-month and 12-month DAPT groups, but also the incidence of definite and definite or probable thrombosis was numerically lower in the 6-month group (Figures 2 and 3). Major bleeding events were numerically more frequent in the 12-month DAPT group, but did not significantly differ compared with the 6-month DAPT group. The incidences of clinical events in the period from 6 to 12 months are shown in Table 4 while the patients were off-DAPT/on-DAPT in the 6-month and 12-month DAPT groups, respectively. No differences were observed between groups in this period.
Outcomes at 12 Months of Follow-up
| 6-month DAPT (n = 1286) | 12-month DAPT (n = 1286) | P | |
|---|---|---|---|
| Primary endpoint | |||
| Cardiac death, myocardial infarction, revascularization or BARC ≥ 3 bleeding | 65 (5.0) | 85 (6.6) | .09 |
| Secondary endpoints | |||
| All-cause death | 21 (1.6) | 24 (1.9) | .4 |
| Cardiac death | 11 (0.9) | 13 (1.0) | .9 |
| Myocardial infarction | 10 (0.85) | 13 (1.00) | .8 |
| Revascularization | 41 (3.2) | 55 (4.3) | .1 |
| Definite thrombosis | 3 (0.24) | 5 (0.40) | .5 |
| Definite and probable thrombosis | 6 (0.5) | 9 (0.7) | .4 |
| BARC ≥ 3 bleeding events | 10 (0.8) | 18 (1.4) | .2 |
BARC, Bleeding Academic Research Consortium; DAPT, dual antiplatelet therapy.
Data are expressed as No. (%).
Outcomes From 6 to 12 Months
| 6-month DAPT (n = 1272)* | 12-month DAPT (n = 1270)* | P | |
|---|---|---|---|
| Primary endpoint | |||
| Cardiac death, myocardial infarction, revascularization or BARC ≥ 3 bleeding | 38 (2.9) | 54 (4.2) | .1 |
| Secondary endpoints | |||
| All-cause death | 7 (0.55) | 8 (0.60) | .8 |
| Cardiac death | 3 (0.23) | 4 (0.31) | .8 |
| Myocardial infarction | 3 (0.23) | 5 (0.40) | .8 |
| Revascularization | 32 (2.5) | 44 (3.5) | .2 |
| Definite thrombosis | 0 (0.0) | 0 (0.0) | |
| Definite and probable thrombosis | 2 (0.15) | 2 (0.15) | .9 |
| BARC ≥ 3 bleeding events | 2 (0.15) | 5 (0.40) | .5 |
BARC, Bleeding Academic Research Consortium; DAPT, dual antiplatelet therapy.
Data are expressed as No. (%).
Subgroup analyses revealed no significant interactions. Of note, in the subgroup of patients with or without ST-segment elevation myocardial infarction, which comprised 15% of patients in the 6-month DAPT group and 16.2% in the 12-month DAPT group, the primary endpoint was met in 7.7% and 8.1%, respectively (P = .8).
DISCUSSIONThe results of this study suggest that, in selected patients (representing around 40% of DES-treated patients), a 6-month DAPT regimen seems to be as safe as a 12-month DAPT regimen from the perspective of ischemic event rates.
The use of DES significantly reduces the need for repeat coronary revascularization. Newer generation DES, particularly everolimus-eluting stents, are linked with reductions in thrombosis rates compared with first-generation DES, or even with BMS.3–7 Therefore, a major limitation for the use of DES resides in the need for a longer-term DAPT regimen as opposed to the 1-month DAPT regimen after BMS implantation. Long-term DAPT is associated with higher bleeding risk and greater cost. These limitations explain why, in some settings with no apparent restrictions on DES use, up to 15% to 20% of patients seem not to benefit from DES, especially amongst the elderly population.
Several trials have compared a short period of 3 to 6 months with a longer period of 12 to 24 months.10–16 A pooled analysis of the first 4 trials published evidence of the absence of a significant difference in ischemic events between the shorter and longer periods but the incidence of bleeding was higher in the longer period.17 Three additional trials addressing short vs long DAPT periods have been presented very recently.14–16 The SECURITY trial14 compared 6-month vs 12-month DAPT in 1399 low-risk patients treated with second-generation DES in stable or unstable angina (infarction as indication for percutaneous coronary intervention was excluded). No differences were observed for any of the clinical endpoints at 12 months. The main limitation was the sample size, low protocol adherence (34% of the patients allocated to 6 months continued DAPT after 6 months) and the inclusion of anatomically low-risk patients. In the ITALIC trial,15 1894 patients with demonstrated nonresistance to acetylsalicylic acid were randomized to 6-month vs 24-month DAPT after implantation of a Xience®. stent. No differences were found for any of the clinical endpoints, including bleeding complications. Finally, the ISAR-SAFE trial,16 which has not yet been published, was planned to recruit 6000 patients but was interrupted after the inclusion of 4000 patients. These were randomized to 6-month or 12-month-DAPT after implantation of DES (89% new-generation). Again, no differences were observed in any of the efficacy or safety endpoints.
However, these studies have some limitations. Being clinical trials, their clinical representativeness is limited, protocol adherence was inadequate,10,14 first-generation DES were used,10,11,16 as well as BMS,10 acetylsalicylic acid resistance was pretested,15 and the first version of zotarolimus-eluting stents, with late lumen loss close to that of BMS, were widely or even exclusively used.10,12,13
The large DAPT trial25 evaluated a longer than 12-month DAPT period after DES implantation. In that study, patients with an uneventful 12-month period after percutaneous coronary intervention were randomized to discontinuation of DAPT at that time or to an extended period of DAPT (up to 30 months). Patients treated with first- and second-generation DES were included. The longer DAPT period (30 months) resulted in a decrease of cardiac adverse events but in an increase in bleeding compared with the 12-month period. However, the DES type reached interaction for the endpoint (hazard ratio = 0.52 for the paclitaxel-eluting stent and hazard ratio = 0.89 for the everolimus-eluting stent with P = .048 for the interaction).
The clinical registries assessing thrombosis risk after early DAPT withdrawal are also limited by their retrospective design.8,9 Various biases are notable, as treatment withdrawal could have been decided following careful consideration of the risk of thrombosis. There is a lack of large prospective registries from real-world practice evaluating shorter DAPT periods.
Finally, an important feature limiting the applicability of a shorter term DAPT regimen is that patients in the setting of an unstable coronary event (most patients undergoing percutaneous coronary intervention nowadays) benefit from a 12-month period of DAPT compared with a 1-month treatment period.18,19 However, we do not know whether this benefit is maintained when a 12-month regimen is compared with a 6-month regimen. Therefore, the discussion remains open and appropriately designed prospective trials and/or registries using current-generation DES are warranted.
Following these considerations, we sought to design this multicenter prospective ESTROFA-DAPT registry evaluating a 6-month DAPT regimen. The inclusion criteria were selected to incorporate mainly patients with stable coronary disease. Regarding unstable patients, we only included those with unstable angina and no rise in cardiac markers. We also included patients with ST-segment elevation myocardial infarction or non—ST-segment elevation myocardial infarction with a bleeding risk that did not necessarily obviate the need for DES in favor of a BMS option. Several in-hospital bleeding risk scores are available for use in patients with ACS but these algorithms were not designed or standardized to predict bleeding risk in the setting of long-term DAPT. Therefore, this decision was left to the discretion of the investigators to consider the balance between restenosis and bleeding risk. Regarding the procedure, patients with stents for left main coronary lesions, as well as bifurcations treated with a 2-stent strategy or patients requiring more than 3 stents were excluded, given the considerably higher risk of stent thrombosis in these cases10.
To be able to effectively compare the results of our series of 6-month vs 12- month DAPT cohorts, we utilized the 12-month DAPT cohort from the ESTROFA-2 study database.22 This previously published registry included 4768 prospectively-enrolled patients treated with second-generation DES and, among them, 4354 (91%) treated with a 12-month DAPT regimen according to the guidelines existing at that time.
Two steps were followed to obtain comparable series of patients from these registries. First, patients in ESTROFA-2 presenting exclusion criteria from ESTROFA-DAPT were excluded (specifically those with treated left main coronary lesions, bifurcations treated with 2 stents, patients with more than 3 stents implanted, and those with previous history of late DES thrombosis). Second, a propensity score matching process was conducted.
Finally, 2 comparable groups across all clinical and procedural characteristics were included in the outcome analysis. Only nonfirst generation DES were included in both registries with a similar proportion for everolimus-eluting stents. However, the zotarolimus-eluting stent model differed, with Endeavor® being used in 12-month DAPT (ESTROFA-2) and Resolute® in 6-month DAPT (ESTROFA-DAPT). The Endeavor® stent, the first version of zotarolimus-eluting stents, showed a late lumen loss close to that of BMS (0.6mm) whereas the Resolute® stent showed a late lumen loss of around 0.15mm. In fact, the RESET and OPTIMIZE trials found no difference between 3-month and 12-month DAPT with the use of the Endeavor® stent.12,13 Therefore, this differential factor could have negatively influenced the results in the 6-month DAPT group but this seems not to have been the case. Therefore, this difference supports the results of the 6-month DAPT approach.
LimitationsAn important limitation of our study is the lack of randomization. Registries entail the problem of bias secondary to known and unknown confounding factors not always accounted for following careful statistical adjustment with matched analyses such as the propensity score.
Nevertheless, although randomized trials are the most appropriate design to compare treatments, registries are still an important source of knowledge and information given the well recognized caveats of randomized trials, such as cost limiting sample sizes, restrictive inclusion beyond exclusion criteria, nonindependent research or no “full real-practice” patient management and follow-up.
A second limitation is the relatively small sample size of various subgroups, especially the ACS subgroup, which does not allow firm conclusions to be drawn regarding short-term DAPT safety in those settings. Another limitation is the nature of patient selection. As described in the “Methods”, the study was mainly safety driven. Those subgroups with a demonstrated benefit following 12 months of DAPT (patients with ACS with no high bleeding risk)18,19 and those with a higher risk of thrombosis and showing a trend for benefit on longer DAPT in trials, were systematically excluded.10
An important consideration is the different time periods for recruitment in both registries. However the potential influence of this time gap could have been attenuated by the following factors: a) New-generation DES only were used in both registries; b) Only patients treated with clopidogrel were included in the analysis. In the more recent ESTROFA-DAPT registry, patients were not treated with new antiplatelet agents by protocol, based on the inclusion criteria (patients under stable clinical conditions or after ACS but with moderately high bleeding risk); c) The methodology of both registries was fairly similar, as previously mentioned in the “Methods”, section.
CONCLUSIONSA 6-month DAPT period following implantation of new-generation DES appears to be noninferior to a 12-month DAPT regimen within the clinical and angiographic contexts evaluated in this multicenter study.
FUNDINGThis study was funded by the Spanish Interventional Cardiology Working Group for Web-based Case Report Forms (CRF) of the Spanish Society of Cardiology.
CONFLICTS OF INTERESTNone declared.