Aortic stenosis (AS) is the most common valvular heart disease in developed countries. The prevalence of AS increases with age and varies from 0.2% at ages 50 to 59 years to 1.3% at 60 to 69 years, 3.9% at 70 to 79 years, and 9.8% at 80 to 89 years.1 Importantly, it is estimated that up to 1.3 million patients in Europe and nearly 1 million patients in the United States will develop severe symptomatic AS by 2025 and that these figures will double by the year 2050. Transcatheter aortic valve implantation (TAVI) has revolutionized the treatment of AS. Worldwide, TAVI is available in more than 65 countries with more than 250 000 implants to date. More than 70 000 cases have been implanted in 2015 and this number is likely to quadruple to over 280 000 by 2025. The adoption of TAVI has been somewhat uneven across different European countries, varying from 160 TAVI units/million inhabitants to 10 to 20 per million. This is determined mainly by local health policy and reimbursement.2 TAVI has become the standard of care for inoperable patients and the preferred treatment option for high-risk patients.
In this article, we discuss the current status of this technique and offer a prediction for what TAVI may hold in the future.
RISK-BASED INDICATIONSIn the American Heart Association/American College of Cardiology and European guidelines,3,4 TAVI is a class I indication for symptomatic patients with severe AS who are not candidates for surgery. Clinical evidence comes from the PARTNER 1B trial. In inoperable patients, a 20% absolute reduction in all-cause mortality was observed from 50.7% with standard medical therapy, including valvuloplasty, to 30.7% when a valve was implanted.5 Importantly, the effect persisted after 5 years of follow-up with no evidence of valve deterioration. The Medtronic Corevalve US Extreme Risk Pivotal trial showed a significantly more favorable 1-year death or stroke rate than that observed in an objective performance goal derived from the conservative arm of PARTNER 1B trial and 5 contemporary balloon aortic valvuloplasty series.6 These results led to rapid Food and Drug Administration approval in this clinical setting.
In severe AS patients who are at high risk of dying or complications after surgery, guidelines recommend that TAVI should be an alternative to conventional surgical valve replacement (Class IIa indication). Clinical evidence for this recommendation mainly comes from 2 trials: PARTNER 1A7 and the Corevalve High-Risk study.8 In the PARTNER 1A trial, TAVI was noninferior to surgery at 1 year, showing no difference in all-cause mortality and, once again, these results were maintained over the following 5 years. The results of the Corevalve High-Risk study showed that TAVI was associated with an all-cause mortality benefit at 1 year. During the 3-year follow-up period, it was demonstrated that TAVI produced a sustained clinical benefit and a lower mean aortic gradient compared with surgery, with no differences in structural valve deterioration, suggesting the use of self-expanding TAVI as the treatment of choice in patients suboptimal for surgery.9
Two studies have recently been published in relation to intermediate-risk patients. In the Transcatheter or Surgical Aortic Valve Replacement in Intermediate-Risk Patients (Society of Thoracic Surgeons [STS] Predicted Risk of Mortality>4%), PARTNER 2 trial, TAVI was similar to surgical aortic valve replacement (SAVR) with respect to the primary endpoint of death or disabling stroke.10 At 2 years, event rates were 19.3% in the TAVI group and 21.1% in the surgery group. When the transfemoral-access cohort was studied separately, TAVI resulted in a lower rate of death from any cause or disabling stroke than did surgery. However, there was no significant between-group difference in the transthoracic-access cohort. A propensity score analysis study has been very recently reported with the SAPIEN 3 valve. The longer-term data in intermediate-risk patients compared outcomes of intermediate-risk patients given a SAPIEN 3 valve or a SAVR.11 For the primary composite endpoint of mortality, stroke, and moderate or severe aortic regurgitation, TAVI was both noninferior (P<.0001) and superior (P<.0001) to SAVR. The authors suggest that TAVI might be the preferred treatment alternative in intermediate-risk patients. With the Medtronic CoreValve System, the SURTAVI trial (recruitment has recently finished) is investigating the safety and efficacy of TAVI in patients with severe, symptomatic AS at intermediate surgical risk by randomizing patients to either SAVR or TAVI. Participants must have comorbidities such that the heart team agrees that the predicted risk of operative mortality is ≥ 3% and<10% at 30 days (Intermediate Clinical Risk classification). Heart team evaluation of clinical surgical mortality risk for each patient includes the calculated STS score for predicted risk of surgical mortality augmented by consideration of the overall clinical status and comorbidities unmeasured by the STS risk calculation. With the available published data and the hopefully positive upcoming results of the SURTAVI trial, we believe that current recommendation for TAVI in intermediate-risk patients will gain evidence in future guidelines.
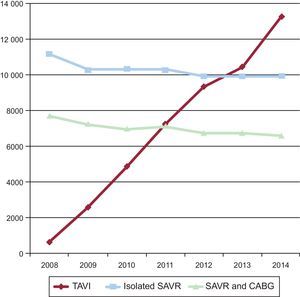
Going one step further in risk-based indications, it is known that the current off-label performance of TAVI in lower-risk patients constitutes a significant proportion of the current practice. Daily practice has overtaken the academic world and there has been a worldwide documented shift in the treatment of AS in the elderly from SAVR to TAVI. Taking Germany—with the highest TAVI penetration rate in Europe—as an example,12 there has been a 20-fold increase in TAVI performance from 2008 to 2014, and the annual number of TAVI procedures already began to surpass that of isolated SAVR from 2013 onward (Figure). Older age was the most frequent reason (70.2%) for the local heart team to select TAVI over SAVR followed by a high predicted surgical risk (53.9%). Interestingly, frailty (46.5%) and patients’ wishes (27.6%) were 2 important reasons for selecting TAVI. Most importantly, in-hospital mortality after TAVI declined from 10.4% in 2008 to 4.2% in 2014 with patients in the lowest risk stratum having the lowest 30-day mortality rate (2%). Length of in-hospital stay remained unchanged for SAVR, but decreased for TAVI over time. In some series, more than one third of patients routinely treated with TAVI had an intermediate-to-low risk, defined as an STS Predicted Risk of Mortality ≤ 8%.
Trends in TAVI, isolated SAVR and SAVR plus CABG in Germany between 2008 and 2014. CABG, coronary artery bypass graft; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation. Adapted from Eggebrecht et al.12 with permission from Europa Digital & Publishing.
The NOTION trial is a randomized clinical trial that compared TAVI with SAVR in an all-comers patient cohort.13 The authors randomly assigned 280 patients with severe AS with low and intermediate surgical risk to receive a self-expanding TAVI or surgical aortic valve replacement. Most of the patients (81.8%) were considered low risk and the mean STS Predicted Risk of Mortality was 3%. The composite primary outcome (death from any cause, stroke, or myocardial infarction at 1 year) and its components were not statistically different between the 2 groups (13.1 vs 16.3% for the composite outcome). TAVI patients had larger improvements in effective valvular orifice area but more frequently required pacemaker implantation, had more aortic valve regurgitation, and worse New York Heart Association functional class at 1 year. Patients treated with SAVR had more major or life-threatening bleeding, cardiogenic shock, acute kidney injury, and new-onset or worsening atrial fibrillation.
Two studies are currently analyzing the role of TAVI compared with SAVR in the treatment of symptomatic patients with severe AS and low risk for surgery. The safety and effectiveness of the SAPIEN 3 transcatheter heart valve in low-risk patients with aortic stenosis (PARTNER 3) trial was designed to establish the safety and effectiveness of the Edwards SAPIEN 3 transcatheter heart valve in patients with severe, symptomatic AS who are at low operative risk for SAVR. The heart team should agree that the patient has a risk of operative mortality<2% (eg, STS Predicted Risk of Mortality<4%). This trial will randomize 1300 patients to TAVI or SAVR. The Medtronic Transcatheter Aortic Valve Replacement in Low-Risk Patients trial will include 1200 patients with 1:1 randomization to receive the Evolut R System or undergo SAVR. The study objective is to demonstrate that the safety and effectiveness of the Medtronic TAVI system as measured by rates of all-cause mortality or disabling stroke at 2 years is noninferior to conventional SAVR in the treatment of severe AS in patients who have a low predicted risk of operative mortality for SAVR. Risk entry inclusion criteria includes documented heart team agreement of low risk for SAVR, where low risk is defined as predicted risk of mortality<3% at 30 days.
PROCEDURE COMPLICATIONSTo expand the TAVI indication to lower-risk patients, procedure complication rates should be the lowest possible. Valve Academic Research Consortium (VARC) statements are a milestone as they have been the standard in defining complications and their applicability. The most important complications in the TAVI procedure are cerebrovascular events, vascular events, cardiac conduction abnormalities, and residual aortic regurgitation (AR).
Cerebrovascular EventsAlthough initially periprocedural stroke or transient ischemic attack was more common after TAVI compared with SAVR at 30 days (5.5% vs 2.4%; P=.04), this difference gradually decreased and by 5 years the difference had dissipated (TAVI, 14.7% vs SAVR, 15.9%). In the more contemporary 2-year results from the CoreValve US pivotal trial (high-risk cohort), the incidence of stroke at 2 years tended to be lower in the TAVI group than in the surgical group (10.9% vs 16.6%; P=.05).8
Vascular ComplicationsWere a frequent early complication of TAVI. In cohort B of the PARTNER trial,5 vascular complications were significantly more common in the TAVI group than in the standard therapy (including percutaneous valvotomy) group (30.7% vs 5%), and in cohort A, vascular complications were more common in the TAVI group than in the SAVR group (17% vs 3.8%). The overall risk of significant vascular damage has decreased with lower profile valve delivery systems but remains above 10%.
Atrioventricular Conduction Defects and Pacemaker ImplantationData on pacing-induced cardiopathy are conflicting.14 Life expectancy among permanent pacemaker recipients, including surgical patients, without significant comorbidity was reported to be comparable with that of the general population. In other studies, however, cardiac pacing was shown to induce electric and mechanical ventricular dyssynchrony, abnormalities in myocardial perfusion, and chronic adverse left ventricular remodeling and to lead to adverse cardiovascular outcomes.
Aortic RegurgitationIt is well established that moderate-to-severe AR after TAVI has adverse effects on mortality. That is why major efforts have been made to diminish this serious complication, including computed tomography valve sizing, use of repositionable and complete recapturable devices, and valves with paravalvular sealing mechanisms. All these measures have achieved a very low rate of moderate-to-severe AR after implantation. An important and unresolved issue is how to quantitate the degree of paravalvular regurgitation. Angiography is usually used during valve implantation, but agreement between angiography and transthoracic echocardiogram (using the VARC-II criteria) in the grading of post-TAVI AR is modest.15 The same poor correlation is obtained when angiography is compared with magnetic resonance. It is then necessary to establish a consensus on how to detect and quantify best regurgitation after TAVI, but it is clear that multimodality imaging techniques as well as hemodynamic analysis should therefore be considered for intraprocedural AR assessment and guidance of the TAVI procedure if there is uncertainty in AR grading.16
FUTURE CHALLENGESFuture expanding indications for TAVI include not only patients who are currently treated with SAVR but also those with off-label indications for other clinical conditions (Table). In this regard, there are some open issues that merit consideration.
Transcatheter Aortic Valve Implantation and “Off-Label” Indications
| Low-risk patients |
| Bicuspid aortic valves |
| Degenerated bioprosthetic surgical aortic valves |
| Severe asymptomatic aortic stenosis |
| Low-flow, low-gradient aortic stenosis |
| Aortic stenosis with severe concomitant cardiac disease (extensive coronary artery disease, mitral regurgitation) |
| Aortic regurgitation in high-risk patients |
Long-term bioprosthetic valve durability is especially important when treating low-risk patients since they are generally expected to live longer than the high-risk group. For comparison, the reported failure rates in surgical bioprostheses are very low,<1% before 5 years and 10% at 10 years for patients older than 65 years. According to the first studies in patients treated with TAVI, 5-year degeneration rates range from 3.5% to 5%.17 The absence of anticoagulation, body mass index, valve-in-valve procedure, and 23mm valve implantation are variables considered independent predictors of valvular degeneration.
The results of the first study that specifically investigated long-term durability in patients undergoing TAVI (with early-generation balloon-expandable devices) has recently been presented at EuroPCR 2016. Dvir et al.,18 evaluated 704 patients (mean age 83 years) who underwent TAVI in 2 hospitals (Canada and France) more than 5 years ago. A total of 378 patients were followed up with repeat echocardiographic examinations for up to 10 years. Patients who died within 30 days of TAVI, with device malfunction immediately after TAVI, and those having valve-in-valve procedures were excluded from the analysis. One hundred patients survived at least 5 years after TAVI and were investigated for valve degeneration, which was defined using central laboratory adjudicated criteria of moderate/severe intravalvular regurgitation and/or AS (mean gradient>20mmHg) that did not appear within 30 days after the TAVI procedure. During the follow-up period, the investigators identified 35 cases of valve degeneration (23 patients with regurgitation and 12 with stenosis/mixed). Renal failure was the strongest correlate of valve degeneration. The Kaplan-Meier estimate for the 8-year rate of structural valve degeneration was approximately 50%. This study recommends that the risk for structural valve degeneration after TAVI should be considered, especially when treating relatively young patients and those at lower surgical risk.
Adjunctive Antithrombotic TherapyThere is no robust evidence to support or refute the use of antiplatelet therapy after TAVI and reports of subclinical leaflet thrombosis19,20 have sparked renewed interest in the most appropriate antithrombotic therapy during and after TAVI. Many trials are trying to answer these questions by testing different drugs and regimens: Bivalirudin vs unfractionated heparin (BRAVO Trial), aspirin vs aspirin+clopidogrel (ARTE Trial), aspirin+clopidogrel vs acenocumarol (AUREA Trial), aspirin vs aspirin+clopidogrel vs oral anticoagulation+clopidogrel (POPular-TAVI-Trial), rivaroxaban+aspirin vs aspirin+clopidogrel (GALILEO Trial) and apixaban vs aspirin+clopidogrel or oral anticoagulation (ATLANTIS trial).
CONCLUSIONSTAVI is a disruptive technology that has revolutionized the treatment of severe symptomatic AS. It has become the “standard of care” for the treatment of high-risk and surgically inoperable patients with symptomatic AS. According to the latest trial results, it will become an alternative for the treatment of patients at intermediate surgical risk. Subgroup analysis suggests that TAVI could also be a therapeutic option in low-risk patients, but only dedicated trials could prove this hypothesis. The durability of TAVI valves represents a crucial point in decision-making about the treatment of younger patients. Finally, cost-effectiveness evaluation is mandatory before the adoption of this new treatment option, particularly in times of continuous growth of health expenditure.
During the drafting of this article and applying to patients with severe AS and intermediate surgical risk, the CoreValve (August 1, 2016) and the XT and SAPIEN 3 valves (September 23, 2016) achieved the CE mark approval. The Food and Drug Administration also approved the use of the XT and SAPIEN3 valves (August 18, 2016).
CONFLICTS OF INTERESTC. Morís is a proctor for Medtronic CoreValve for the valve EVOLUT R.