Human immunodeficiency virus (HIV) infection has spread across all 4 corners of the globe and is one of the most deadly infections in the past century. Worldwide, it is estimated that approximately 34 million people are currently living with HIV of whom 90% are in developing countries.
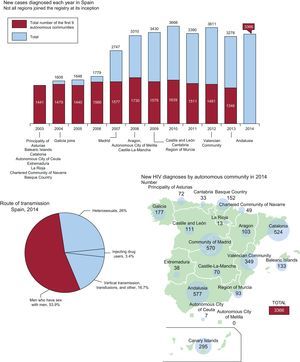
In 2014 in Spain 113 509 people were diagnosed and living with HIV, of whom 104 769 (92.3%) received antiretroviral therapy (ATR).1 That same year, 3366 people were notified of new HIV diagnoses, representing an HIV diagnosis rate of 7.25 per 100 000 persons/year. This rate presented differently means 10 new HIV diagnoses were made every day in Spain that year. In 2014, men accounted for most new HIV diagnoses (84.7%). Median age at diagnosis was 35 years, although people aged 50 years and older accounted for 13.4% of new diagnoses. The sexual route of transmission was the most frequently reported accounting for 80% of transmissions: 54% in homosexuals and 26% in heterosexuals. Nearly half the new diagnoses (46.2%) had a delay in detection, particularly among people who inject drugs (75%) and heterosexual men (58.6%). Increasing substantially with age, this delay was higher in those aged 50 years or older. The proportions of new diagnoses from people born outside Spain have declined from 40% in 2009 to 32.2% in 2014. The most common source regions were Latin America and sub-Saharan Africa (Figure).1
Temporal incidence of HIV in Spain (data from Spanish Department of Health, Social Services and Equality1,2). HIV, human immunodeficiency virus.
The HIV and acquired immune deficiency syndrome (HIV/AIDS) was responsible, between 1981 and 2013, for 56 829 deaths in Spain, 81.0% in men and 19.0% in women. The number of deaths peaked at 5857 in 1995. Since then, the number of deaths has substantially decreased, with a 54% reduction between 2003 and 2013.2
In 2013, there were a total of 390 419 deaths in Spain, of which 750 were due to HIV/AIDS (1.9 per 1000 patients/year)2. According to data from the Spanish AIDS Research Network, the overall mortality rate for people living with HIV was 6.8 (95% confidence interval, 5.9-7.9) times higher than the same age and same sex general population.3
CARDIOVASCULAR RISKAntiretroviral therapy is very effective for controlling HIV infection and thus has revolutionized the prognosis and radically increased survival of people living with HIV. However, as HIV-infected populations under ART are living substantially longer, they are increasingly exposed to new emerging health issues, notably chronic diseases. Cardiovascular complications now represent a leading cause of morbidity and mortality in the HIV-infected population, especially in developed countries (6%-15% of mortality).4
Populations living with HIV have a 2-fold risk of cardiovascular disease compared with the general population. Relevant differences in cardiovascular risk profiles in HIV patients are highlighted in numerous studies. Many groups have reported that traditional risk factors, particularly smoking and diabetes, are more prevalent in HIV-infected populations in developed countries.5
HIV and LipidsBoth HIV infection and its treatment are associated with lipid abnormalities. Before ART was available, several studies found that HIV-infected individuals had elevated plasma triglyceride and free fatty acid levels, although they had decreased total cholesterol, high-density lipoprotein-cholesterol, and low-density lipoprotein-cholesterol.6 In addition, first-generation protease inhibitors (PI) and NNRTIs (non-nucleoside reverse transcriptase inhibitors) lead to a substantial increase in total and low-density lipoprotein-cholesterol. Cardiovascular risk in HIV patients was associated with lower levels of small and large high-density lipoprotein-particle concentrations, independently of other cardiovascular risk factors.
HIV and GlucoseAbnormalities in glucose homeostasis in HIV-infected individuals are also frequent. Early articles reported that clinically stable symptomatic HIV-infected men who were participants in euglycemic clamp studies had higher rates of insulin clearance and increased insulin sensitivity in peripheral tissues than did the uninfected control group. The increase in noninsulin-mediated glucose uptake observed in HIV patients has been accounted for by an increase in nonoxidative glucose disposal. Glucose production from the liver tends to increase, but glucose cycling does not change. Although many studies link the use of first-generation PIs to the development of insulin resistance, there is also evidence suggesting that insulin resistance may also have an HIV associated component.7
HIV and Arterial HypertensionRegarding arterial hypertension, some early studies suggested a link between PI-based ART and elevated blood pressure. More recent and larger studies, including the Data Collection of Adverse events of Anti-HIV Drugs (D:A:D) study, found that other factors accounted for this relationship including age, race, and increases in body mass index occurring after ART initiation8 in particular. Another study suggested a link between the duration of ART and high blood pressure. Prolonged ART, defined as a duration of 2 to 5 years, was independently associated with the development of hypertension in the Multicenter AIDS Cohort Study study, whereas ART for a duration of < 2 years was not9. Recently, our group observed that nadir CD4 cell count (< 200/μL3) was associated with increased aortic stiffness in HIV-infected individuals after a long-term follow up (> 7 years) reinforcing the complex association between vascular disease and HIV-related factors10 including ART, immunodeficiency, chronic immune activation, and low grade inflammation.
HIV infection may contribute to arterial hypertension directly through immune activation and indirectly through inflammation which provokes endothelial and vascular smooth muscle cell dysfunctions. Chronic immune activation and viral replication may lead to permanent T-cell activation, which may be affected by reactivation of other viruses, for example cytomegalovirus. Furthermore, ART and HIV may also lead to chronic inflammation and both have a complex interaction with coagulation factors. In fact, higher levels of aortic inflammation in HIV-infected individuals have been observed compared with uninfected individuals with the same cardiovascular risk profile. Higher levels of interleukin-6, high-sensitivity C-reactive protein, and D-dimer were associated with increased all-cause mortality and predicted cardiovascular disease independently of other risk factors.4
CORONARY HEART DISEASENumerous studies suggest that the increased risk of acute myocardial infarction (AMI) among HIV-positive people is likely associated with HIV, ART, and the comorbid illness burden including traditional risk factors. Patients with HIV have a 50% increased risk of AMI and more postdischarge adverse cardiac events. The incidence of AMI is 3.5 per 1000 patients/year.8 Possible mechanisms may involve inflammation, CD4 cell count depletion, altered coagulation, dyslipidemia, impaired arterial elasticity, and endothelial dysfunction.11 Antiretroviral therapy is associated with metabolic changes and abnormal fat distribution, which in turn are linked with insulin resistance, diabetes, and dyslipidemia. Although HIV and ART are associated with AMI risk, results from the SMART study12 show that continuous HIV viral suppression provides lower cardiovascular disease risk than drug interruption, suggesting that the virus may play a direct role.
The spectrum of coronary heart disease (CHD) is similar in HIV-infected and HIV-uninfected patients. In published studies, the “typical” patient is a younger man (< 50 years) with a long known duration of HIV (> 8 years), who is taking ART generally including a PI (> 59%), is a smoker (> 45%), and has dyslipidemia (17%-58%). In these studies, the most frequent CHD presentation is ST-segment elevation myocardial infarction (29%-64%), followed by non–ST-segment elevation myocardial infarction (20%-48%) and unstable angina (18%-46%).11 The prognosis of HIV-infected patients during the acute phase of AMI does not differ from uninfected individuals in several studies.13 In the PACS-HIV study,14 recurrent acute coronary events were substantially more frequent in HIV-infected patients than in uninfected patients (hazard ratio = 6.5; 95% confidence interval, 1.7 to 23.9) at 1-year follow up. The treatment of CHD, whether stable or unstable, does not differ between HIV-infected individuals and the general population. Cardiologists must be aware of several potential drug-drug interactions particularly with antiplatelet agents, statins and antiretrovirals (Table). Ticagrelor should not be used with PIs that potentially inhibit the cytochrome P450 3A4 (CYP3A4) pathway as it could increase the area under the curve (AUC) of ticagrelor and thus increase the risk of bleeding.15 Regarding prasugrel, a single dose of 100mg of ritonavir (the most frequently prescribed PI) decreased the AUC of prasugrel in healthy volunteers (38%). The product monograph states that CYP3A inhibitors are not anticipated to have a significant effect on the pharmacokinetics of active metabolite.15 This may be due to ability of multiple additional CYPs to form prasugrel active metabolite. Therefore, prasugrel could be used in patients with concomitant PIs. A weak interaction is expected between clopidogrel and PIs. Efavirenz and etravirine are NNRTIs that could decrease the activity of clopidogrel, suggesting the concomitant use of prasugrel or ticagrelor with this drug. No interactions have been found between nevirapine and rilpivirine and any of the P2Y12 inhibitors. Ticagrelor or prasugrel should be given with etravirine. Cobicistat is frequently associated with new integrase inhibitors and is a potent inhibitor of CYP3A4, therefore the use of prasugrel is preferred (risk of decreased activity with clopidogrel and risk of bleeding with ticagrelor). Finally, the new integrase inhibitors (raltegravir, elvitegravir, dolutegravir) and maraviroc (CCR5 inhibitors) have no potential interaction with any P2Y12 inhibitors. Statins that strongly interfere with the CYP3A4 (lovastatin and simvastatin) are contraindicated with PIs. Non-nucleoside reverse transcriptase inhibitors tend to decrease statin activity and darunavir increases the AUC of pravastatin. Low-dose atorvastatin and rosuvastatin are the statins of choice in secondary prevention.
Interactions Between Antithrombotic Drugs and Antiretroviral Therapy
| Antithrombotic drugs | Antiretroviral therapy |
|---|---|
| Clopidogrel | Weak interaction with PIs and cobicistat (decrease clopidogrel efficacy) and interaction with efavirenz and etravirine. No interaction with oher NNRTis, anti-integrase or maraviroc |
| Ticagrelor | Contraindicated with PIs (risk of bleeding) |
| Prasugrel | Possible use with PIs |
| New oral anticoagulants | Contraindicated with PIs except for dabigatran (slight decrease of AUC in healthy volunteers) |
| Warfarin | Precaution with PIs (decrease plasma levels of warfarin) |
AUC, area under the curve; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
Cardiovascular manifestations of HIV have been transformed since the introduction of ART. On the one hand, ART has significantly modified the course of HIV disease, lengthened survival, and improved the quality of life of HIV-infected patients. On the other hand, early data raise concerns that ART is associated with an increase in peripheral and coronary arterial diseases. This therapy has, however, also been associated with a decrease of other cardiac diseases including pericardial, myocardial, and valvular heart disease.16
PERICARDIAL DISEASEPericardial disease was the most common cardiac manifestation in HIV-infected individuals in the pre-ART era. Pericardial disease remains an important issue in developing countries particularly in sub-Saharan Africa with predominant tuberculosis etiology. Pericarditis and pericardial effusion can be idiopathic, related to opportunistic infections including tuberculosis and malignancies, especially lymphoma and Kaposi's sarcoma.16
MYOCARDIAL DISEASEMyocardial disease presents in 3 forms in patients with HIV: focal myocarditis, echocardiographic evidence of impaired ventricular function, and clinical cardiomyopathy.17 In the pre-ART era, studies on autopsies revealed that myocardial disease prevalence ranged from 9% to 52%, with an average of 33%, mostly asymptomatic. Dilated cardiomyopathy was found in 10% to 20% of echocardiographic studies before the advent of ART. Several explanations have been proposed for myocardial involvement during HIV, including: an autoimmune process induced by infection, direct myocardial infection or other opportunistic infections, ART, cachexia, selenium deficiency, and proinflammatory cytokines, etc. Developed countries have witnessed a marked reduction of myocardial disease since the introduction of ART. In sub-Saharan Africa, however, myocardial diseases remains the leading cause of hospitalization in intensive cardiac care units (> 40%).18
VALVULAR HEART DISEASEHIV itself is a risk factor for infective endocarditis in injecting drug users but not in drug nonusers. In the pre-ART era, the prevalence of infective endocarditis varied from 6% to 34% with predominantly right heart involvement. Nonbacterial thrombotic endocarditis cases have been reported in association with profound immunosuppression and the presence of a wasting syndrome. Kaposi's sarcoma and non-Hodgkin type B lymphoma could be confused with infective endocarditis on echocardiography and must be evoked in atypical endocarditis with negative hemocultures and immunosuppression.16
PULMONARY HYPERTENSIONPulmonary hypertension (PH) is a rare complication of HIV but is more prevalent in HIV-infected populations than in the general population. This complication is observed in 1 in 200 HIV-infected patients and is an independent predictor of mortality. The pathophysiology of HIV-related PH is complex with viral proteins seem to play a major role. Other factors may also contribute, such as coinfection with other microorganisms, HIV-related systemic inflammation, microvascular thrombi or pulmonary embolisms. The clinical presentation and diagnosis of HIV-related PH is similar to those in other forms of PH.16
TUMORSKaposi's sarcoma may compromise the myocardium and/or pericardium, causing pleural effusion and cardiac tamponade in some patients. In the pre-ART era, the incidence of these tumors ranged from 12% to 28% and nowadays they are a rare complication. Non-Hodgkin lymphoma can also occur as a primary cardiac lymphoma, causing heart failure, superior venous cave syndrome, atrioventricular block, or mechanical obstruction to blood flow.16
ARRHYTHMIASCardiovascular involvement in HIV-infected individuals can take the form of nonspecific electrocardiographic abnormalities. Arrhythmias are observed, sometimes in association with tumor involvement or cardiomyopathy but occasionally secondary to therapeutic drugs. Atrial fibrillation is rare in HIV-infected populations due to their younger age. Markers of HIV disease severity, both low CD4 cell count and high viral load, have been independently associated with the occurrence of atrial fibrillation.19 QT prolongation and torsade de pointe has been described in HIV-infected patients even in the absence of antiretroviral drugs. It has, however, been observed that pentamidine—directly or secondary to its hypomagnesemia effect—or with PIs—can cause long QT syndrome by blocking human ether-à-go-go-related gene (HERG) channels.16 Systolic and diastolic left ventricular dysfunction, particularly in HIV-infected individuals with a high viral load, has been associated with sudden cardiac death.20
MANAGING RISK FACTORS AND PREVENTION OF CARDIOVASCULAR DISEASEThe prevention of cardiovascular diseases has now become a fundamental part of the routine management of HIV-infected individuals. Cardiovascular risk factors should be reduced whenever possible. Current strategies to decrease the risk of CHD include early initiation of ART regimens with the fewest metabolic adverse effects, and careful management of traditional cardiovascular risk factors through both nonmedical and medical treatments. Future strategies to prevent CHD in HIV-infected individuals may involve the use of HIV-tailored cardiovascular risk-prediction paradigms and the administration of therapies alongside ART that could further decrease proatherogenic HIV-specific immune activation. Many ongoing trials are currently evaluating the benefits of modulating immune activation and the associated vascular risk using methotrexate, canakinumab (interleukin-1β monoclonal antibody), tocilizumab (interleukin-6 monoclonal antibody), and probiotics, etc.
Given the high prevalence of traditional risk factors for cardiovascular disease in HIV-infected populations, it has become the first priority to ensure management of dyslipidemia and hypertension and to provide counselling for behavior changes including smoking cessation and reduction and discontinuation of illicit drug use. Management of these cardiovascular risk factors can be done according to guidelines established for uninfected individuals,21 with a few exceptions due to potential lethal drug-drug interactions (see above for antiplatelet agents and statins). Antihypertensive drugs, particularly dihydropiridine calcium-channel-blockers, may interact with PIs and should be given at a low dose. Aspirin in primary prevention should be prescribed for high risk patients (eg Framingham risk score ≥ 20% and with controlled hypertension) as recommended for the general population.
CONCLUSIONSCardiovascular disease associated with HIV is caused by a complex network interaction of factors associated with the virus, the host, and ART. Appropriate therapy is guided by balancing the risks and benefits of each and by knowledge of the pathophysiological mechanisms involved. In the years to come, both the risk and the prevalence of cardiovascular disease associated with HIV will continue to increase due to the aging of this specific population. Consequently, preventive measures must now be strengthened to mitigate cardiovascular risk.
Recently, studies have shown a decrease in the incidence of myocardial infarction compared with the general population, possibly due to better preventive strategies being used in these HIV infected populations. Early ART initiation may reduce cardiovascular complications but this is yet to be demonstrated. New antiretroviral agents have less impact on metabolic disorders and therefore probably fewer cardiovascular risk. However, the massive HIV-related benefits of all antiretroviral drugs must be put into perspective as they largely outweigh the cardiovascular risk. Across developed countries today the choice of antiretroviral drug combination must be made on an individualized basis for each patient and must reflect the patient's adherence to therapy, cardiovascular risk, and comorbidities.4 Optimally coordinated care between cardiologists and HIV physicians is paramount for the chronic disease management of HIV and to further improve the prognosis of HIV-infected individuals.
CONFLICTS OF INTERESTNone declared.
We thank Mabel Nuernberg, MSc, for editorial support.