There is limited evidence on procedural and clinical outcomes in patients treated with overlapping bioresorbable scaffolds vs overlapping everolimus-eluting stents. We evaluated the outcomes of propensity-matched patients treated with overlapping scaffolds vs everolimus-eluting stents.
MethodsAfter propensity matching, 70 consecutive stable angina patients treated with overlapping bioresorbable scaffolds and 70 patients treated with overlapping new generation everolimus stents were included in this study. The primary outcome was the 1-year rate of major adverse cardiovascular events, defined as the composite of all-cause mortality, nonprocedural myocardial infarction, and target-vessel revascularization.
ResultsPatients in the 2 groups had similar age (scaffold vs stent: 64.5 ± 10.3 vs 66 ± 9.7 years; P=.381), sex, diabetes, previous cardiovascular history, and SYNTAX score (scaffold vs stent: 18.6 ± 9.2 vs 19.4 ± 10.4; P=.635). Postprocedural acute gain was significantly lower in patients treated with scaffolds (1.82±0.66 vs 2.03±0.68mm; P=.033). At 1-year follow up, the estimated major adverse cardiovascular event rate was not significantly different between the 2 groups (scaffold vs stent: 14.5% vs 14.6%; Plog-rank=.661). Similarly, no significant differences were seen in 1-year rates of target vessel (scaffold vs stent: 14.5% vs 10%; Plog-rank=.816) or target lesion revascularization (scaffold vs stent: 9.7% vs 8.3%; Plog-rank=.815).
ConclusionsTreating long lesions with overlapping scaffolds is feasible with acceptable 1-year outcomes.
Keywords
The initial experience with bioresorbable scaffold (BRS) implantation in de novo simple lesions has been promising with acceptable long-term outcomes in the ABSORB cohort A.1 The ABSORB2 multi-imaging modality study revealed unchanged late lumen loss after the first year of Absorb v1.1 implantation, whereas on intravascular ultrasound (IVUS), mean lumen, scaffold and vessel area showed enlargement up to 2 years.
Even though the emerging randomized data3-8 suggest similar angiographic and intermediate-term clinical outcomes in patients treated with BRS and those treated with new generation everolimus-eluting stents (EES), real-world studies have revealed some worrying signs of increased rates of scaffold thrombosis in patients treated with BRS. The 6-month 3.0%, 2.2%, 2.1% scaffold thrombosis rates quoted in the AMC, BVS EXPAND and GHOST-EU registries,9 respectively, cannot be considered negligible and several potential scaffold thrombosis mechanisms have been proposed, one of which involves overlapping segments. Previous studies in porcine models10 revealed reduced endothelial coverage of stacked BRS struts 28 days post implantation, suggesting a potential substrate for scaffold thrombosis and future target lesion revascularization (TLR).
The current study sought to evaluate procedural (acute gain, angiographic, and procedural success) and mid-term clinical outcomes among stable angina patients treated with percutaneous coronary intervention with overlapping BRS or new generation EES.
METHODSA total of 590 stable angina patients were treated at the EMO GVM Centro Cuore Columbus, Milan, Italy with BRS (Absorb v1.1, Abbott Vascular; Santa Clara, California, United States) from May 2012 to July 2014 or new generation durable polymer EES (XIENCE Prime, Abbott Vascular or Promus Element, Boston Scientific; Natick, Massachusetts, United States), from May 2011-July 2014. Of these, 219 were treated with BRS and 371 with new generation EES. There were no particular selection criteria for the implantation of BRS vs EES in our population other than patient preference. A vessel reference vessel diameter>4.2mm or<2.5mm was prohibitive for BRS implantation. Overlapping lesions (Figures 1 and 2) were identified as those requiring implantation of at least 2 overlapping stents/scaffolds, excluding bifurcation lesions treated with a 2-stent/scaffold strategy. Other exclusion criteria included acute coronary syndrome (ACS) presentation and end-stage renal failure (on hemodialysis).
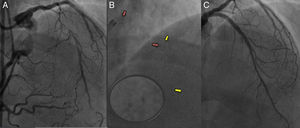
Overlapping proximal and mid segment bioresorbable scaffolds (BRS) in a young patient with a chronic total occlusion of the left anterior descending. A: left anterior descending backfilling from collaterals from the distal right. Long segment of chronic total occlusion seen in ostial-proximal segment. B: after aggressive lesion preparation, 2 BRS (3.5 × 18mm Absorb) were implanted at the ostial-proximal and mid segments with minimal overlap. The platinum markers of the proximal BRS are shown with the red arrows, while the platinum markers of the mid segment BRS are shown with the yellow arrows. At the overlap segment, the distal platinum marker of the proximal BRS and the proximal platinum marker of the distal BRS (enlarged circle) can be seen side by side. C: final result after postdilatation of the proximal-mid segment BRS and drug eluting balloon at the distal segment.
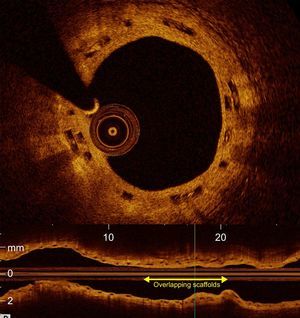
Optical coherence tomography (cross-section and longitudinal images) of a patient treated with overlapping bioresorbable scaffolds (∼15mm overlap) at the 2-year follow-up. Scaffold struts can been seen stacked on each other (black boxes) and fully covered by neointima. In the longitudinal cross-section, the length of the overlap is shown with the yellow arrow.
A total of 109 stable angina patients treated with overlapping BRS and 149 patients treated with overlapping new generation EES were included in the analysis. All patients provided written informed consent, according to the Declaration of Helsinki. All clinical data at follow-up were collected from hospital visits or telephone consultations for all patients.
All patients were pretreated with aspirin and clopidogrel, ticagrelor or prasugrel and were instructed to continue with dual antiplatelet therapy for at least 1 year. Quantitative coronary angiographic measurements were performed offline using a validated edge detection system (CMS, version 5.2, Medis Medical Imaging Systems BV; Leiden, The Netherlands) by an expert operator; pre- and postprocedural minimal lumen diameter and the percentage of diameter stenosis were measured at baseline. The in-stent/scaffold acute gain was defined as the difference between pre- and postprocedural minimal lumen diameter. Stent overlap was defined as the presence of ≥ 2 stents within a single treated lesion, as determined by quantitative coronary angiography.11 For patients presenting with TLR, overlapping stent zones were identified based on the position of the stent balloon markers of the second stent relative to the first stent. In particular, the overlapping segments were characterized as adjacent scaffolds/stents (overlap<1mm), minimal (1-2mm) and complete (>2mm overlap). Intravascular ultrasound was used in the majority of BRS cases to ensure optimal expansion and apposition of the scaffold. The fairly high use of IVUS among patients with EES reflects the complexity of the lesions treated (calcified, in-stent restenosis, bifurcations etc).
Furthermore, lesions with TLR were categorised using the Mehran classification.12 Angiographically moderate/severe calcification was defined as radiopacities noted with or without cardiac motion before contrast injection, generally compromising both sides of the arterial lumen.13,14 The SYNTAX score was prospectively calculated for all patients.13 Angiographic success was defined as a minimum diameter stenosis of<20%, with TIMI flow grade 3 without occlusion of a significant side branch, flow-limiting dissection, distal embolization, or angiographic evidence of thrombus. Procedural success was defined as the composite endpoint of angiographic success without associated in-hospital major clinical complications (ie, death, myocardial infarction [MI], stroke, or emergency coronary artery bypass [CABG]).15 Periprocedural myocardial infarction (PMI) definition was similar to that used in the study by Vranckx et al.16
The primary endpoint was the 1-year rate of major acute cardiovascular events (MACE), defined as the composite of all-cause mortality, nonprocedural MI, and target vessel revascularization (TVR). Secondary endpoints included procedural outcomes (acute gain, angiographic and procedural success), TLR and TVR. TVR was defined as repeat revascularization of the target vessel, and TLR was defined as repeat revascularization of the stented segment, or within 5mm from the stent edges. Nonprocedural follow-up acute MI was defined as per current guidelines.17 Stent thrombosis was classified according to the Academic Research Consortium definition.18
Statistical AnalysisAll continuous variables were tested for normality using the Kolmogorov-Smirnov test. Continuous variables are presented as mean ± standard deviation or median [interquartile range] for normally and not normally distributed variables, respectively. Differences in continuous variables between overall cohort groups were analysed using the Student t-test or Mann-Whitney U test.
To reduce the effect of selection bias and other baseline confounding in this retrospective study, we performed propensity score matching (BRS:EES, 1:1). The propensity scores were estimated with the use of a nonparsimonious multivariable logistic regression model, with percutaneous intervention with BRS or EES as the dependent variable, and the following patient and angiographic characteristics as covariates (considered clinically important predictors of MACE): age, sex, diabetes mellitus, prior MI, prior percutaneous coronary intervention, prior CABG, SYNTAX score, and total stent length. Matching was performed with the use of a 1:1 matching protocol without replacement (greedy matching algorithm), with caliper width equal to 0.2 of the standard deviation of the logit of the propensity score. After propensity score matching, all of the standardized differences for each of the baseline variables were<0.10 (10%). After propensity matching, differences in continuous variables were analyzed using the paired t-test. Categorical variables are expressed as numeric values and percentages. Categorical data were compared using the chi-square or Fisher exact tests (overall cohort) or McNemar test (propensity-matched cohort). The cumulative incidences were generated using Kaplan-Meier analysis, and the significance of observed differences was assessed with the log-rank test (overall cohort) or with the use of a Cox proportional-hazards regression model that was stratified on the matched pair to preserve the benefit of matching (propensity matched cohort).
All reported P values were 2-sided, and values of P<.05 were regarded as statistically significant. Analyses were performed using SPSS (version 21.0, IBM Corp.; Armonk, New York, United States).
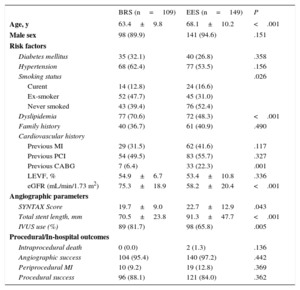
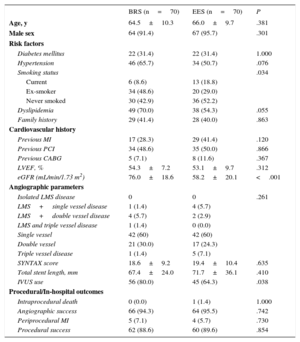
RESULTSBaseline CharacteristicsPatient DemographicsBaseline characteristics of the overall overlap cohort are presented in Table 1. Of these, 70 patients treated with BRS were matched 1:1 with 70 EES patients. Baseline characteristics of the propensity matched cohort are presented in Table 2. None of the propensity-matched variables showed any statistically significant differences between the 2 groups. Patients treated with BRS were more often ex-smokers, while patients treated with EES had a significantly lower estimated glomerular filtration rate (Table 2). Single-vessel disease was present in 60% of the patients in both groups. Intravascular ultrasound was more commonly used among patients treated with BRS.
Baseline Characteristics in the Overall Cohort
| BRS (n=109) | EES (n=149) | P | |
|---|---|---|---|
| Age, y | 63.4±9.8 | 68.1±10.2 | <.001 |
| Male sex | 98 (89.9) | 141 (94.6) | .151 |
| Risk factors | |||
| Diabetes mellitus | 35 (32.1) | 40 (26.8) | .358 |
| Hypertension | 68 (62.4) | 77 (53.5) | .156 |
| Smoking status | .026 | ||
| Curent | 14 (12.8) | 24 (16.6) | |
| Ex-smoker | 52 (47.7) | 45 (31.0) | |
| Never smoked | 43 (39.4) | 76 (52.4) | |
| Dyslipidemia | 77 (70.6) | 72 (48.3) | <.001 |
| Family history | 40 (36.7) | 61 (40.9) | .490 |
| Cardiovascular history | |||
| Previous MI | 29 (31.5) | 62 (41.6) | .117 |
| Previous PCI | 54 (49.5) | 83 (55.7) | .327 |
| Previous CABG | 7 (6.4) | 33 (22.3) | .001 |
| LEVF, % | 54.9±6.7 | 53.4±10.8 | .336 |
| eGFR (mL/min/1.73 m2) | 75.3±18.9 | 58.2±20.4 | <.001 |
| Angiographic parameters | |||
| SYNTAX Score | 19.7±9.0 | 22.7±12.9 | .043 |
| Total stent length, mm | 70.5±23.8 | 91.3±47.7 | <.001 |
| IVUS use (%) | 89 (81.7) | 98 (65.8) | .005 |
| Procedural/In-hospital outcomes | |||
| Intraprocedural death | 0 (0.0) | 2 (1.3) | .136 |
| Angiographic success | 104 (95.4) | 140 (97.2) | .442 |
| Periprocedural MI | 10 (9.2) | 19 (12.8) | .369 |
| Procedural success | 96 (88.1) | 121 (84.0) | .362 |
BRS, bioresorbable scaffold; CABG, coronary artery bypass surgery; EES, everolimus-eluting stents; eGFR, estimated glomerular filtration rate; IVUS, intravascular ultrasound; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Baseline Characteristics in the Propensity Matched Groups
| BRS (n=70) | EES (n=70) | P | |
|---|---|---|---|
| Age, y | 64.5±10.3 | 66.0±9.7 | .381 |
| Male sex | 64 (91.4) | 67 (95.7) | .301 |
| Risk factors | |||
| Diabetes mellitus | 22 (31.4) | 22 (31.4) | 1.000 |
| Hypertension | 46 (65.7) | 34 (50.7) | .076 |
| Smoking status | .034 | ||
| Current | 6 (8.6) | 13 (18.8) | |
| Ex-smoker | 34 (48.6) | 20 (29.0) | |
| Never smoked | 30 (42.9) | 36 (52.2) | |
| Dyslipidemia | 49 (70.0) | 38 (54.3) | .055 |
| Family history | 29 (41.4) | 28 (40.0) | .863 |
| Cardiovascular history | |||
| Previous MI | 17 (28.3) | 29 (41.4) | .120 |
| Previous PCI | 34 (48.6) | 35 (50.0) | .866 |
| Previous CABG | 5 (7.1) | 8 (11.6) | .367 |
| LVEF, % | 54.3±7.2 | 53.1±9.7 | .312 |
| eGFR (mL/min/1.73 m2) | 76.0±18.6 | 58.2±20.1 | <.001 |
| Angiographic parameters | |||
| Isolated LMS disease | 0 | 0 | .261 |
| LMS+single vessel disease | 1 (1.4) | 4 (5.7) | |
| LMS+double vessel disease | 4 (5.7) | 2 (2.9) | |
| LMS and triple vessel disease | 1 (1.4) | 0 (0.0) | |
| Single vessel | 42 (60) | 42 (60) | |
| Double vessel | 21 (30.0) | 17 (24.3) | |
| Triple vessel disease | 1 (1.4) | 5 (7.1) | |
| SYNTAX score | 18.6±9.2 | 19.4±10.4 | .635 |
| Total stent length, mm | 67.4±24.0 | 71.7±36.1 | .410 |
| IVUS use | 56 (80.0) | 45 (64.3) | .038 |
| Procedural/In-hospital outcomes | |||
| Intraprocedural death | 0 (0.0) | 1 (1.4) | 1.000 |
| Angiographic success | 66 (94.3) | 64 (95.5) | .742 |
| Periprocedural MI | 5 (7.1) | 4 (5.7) | .730 |
| Procedural success | 62 (88.6) | 60 (89.6) | .854 |
BRS, bioresorbable scaffold; CABG, coronary artery bypass surgery; EES, everolimus-eluting stents; eGFR, estimated glomerular filtration rate; IVUS, intravascular ultrasound; LMS, left main stem; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Data are expressed as No. (%) or mean±standard deviation.
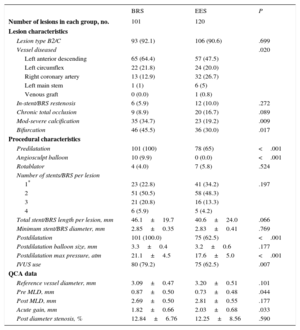
Rates of type B2/C lesions were similar in the 2 groups (Table 3). The left anterior descending artery was more frequently treated in the BRS group than in the EES group, whereas the right coronary artery was more frequently treated in the EES group. There was a significantly higher rate of calcific and bifurcation lesions among patients treated with BRS whereas there was a trend for higher rates of chronic total occlusions and in-stent restenosis in the EES group (Table 3). SYNTAX scores were similar between the 2 groups (Table 2). Predilatation (with both conventional and scoring balloons) and postdilatation were more frequently performed in patients treated with BRS (Table 3). Out of 101 lesions treated in the BRS group, 78 involved overlapping segments, whereas out of 120 lesions in the EES group, 79 involved overlapping segments (Table 3).
Lesion and Procedural Characteristics in the Propensity Matched Groups
| BRS | EES | P | |
|---|---|---|---|
| Number of lesions in each group, no. | 101 | 120 | |
| Lesion characteristics | |||
| Lesion type B2/C | 93 (92.1) | 106 (90.6) | .699 |
| Vessel diseased | .020 | ||
| Left anterior descending | 65 (64.4) | 57 (47.5) | |
| Left circumflex | 22 (21.8) | 24 (20.0) | |
| Right coronary artery | 13 (12.9) | 32 (26.7) | |
| Left main stem | 1 (1) | 6 (5) | |
| Venous graft | 0 (0.0) | 1 (0.8) | |
| In-stent/BRS restenosis | 6 (5.9) | 12 (10.0) | .272 |
| Chronic total occlusion | 9 (8.9) | 20 (16.7) | .089 |
| Mod-severe calcification | 35 (34.7) | 23 (19.2) | .009 |
| Bifurcation | 46 (45.5) | 36 (30.0) | .017 |
| Procedural characteristics | |||
| Predilatation | 101 (100) | 78 (65) | <.001 |
| Angiosculpt balloon | 10 (9.9) | 0 (0.0) | <.001 |
| Rotablator | 4 (4.0) | 7 (5.8) | .524 |
| Number of stents/BRS per lesion | |||
| 1* | 23 (22.8) | 41 (34.2) | .197 |
| 2 | 51 (50.5) | 58 (48.3) | |
| 3 | 21 (20.8) | 16 (13.3) | |
| 4 | 6 (5.9) | 5 (4.2) | |
| Total stent/BRS length per lesion, mm | 46.1±19.7 | 40.6±24.0 | .066 |
| Minimum stent/BRS diameter, mm | 2.85±0.35 | 2.83±0.41 | .769 |
| Postdilatation | 101 (100.0) | 75 (62.5) | <.001 |
| Postdilatation balloon size, mm | 3.3±0.4 | 3.2±0.6 | .177 |
| Postdilatation max pressure, atm | 21.1±4.5 | 17.6±5.0 | <.001 |
| IVUS use | 80 (79.2) | 75 (62.5) | .007 |
| QCA data | |||
| Reference vessel diameter, mm | 3.09±0.47 | 3.20±0.51 | .101 |
| Pre MLD, mm | 0.87±0.50 | 0.73±0.48 | .044 |
| Post MLD, mm | 2.69±0.50 | 2.81±0.55 | .177 |
| Acute gain, mm | 1.82±0.66 | 2.03±0.68 | .033 |
| Post diameter stenosis, % | 12.84±6.76 | 12.25±8.56 | .590 |
BRS, bioresorbable scaffold; EES, everolimus-eluting stent; MLD, minimal lumen diameter; QCA, quantitative coronary angiography.
Postprocedural acute gain was significantly lower in patients treated with BRS (1.82±0.66 vs 2.03±0.68; P=.033). Angiographic success rates were similar in the 2 groups (BRS vs EES: 94.3% vs 95.5%; P=.742). The prevalence of PMI was also similar (BRS vs EES: 7.1% vs 5.7%; P=.73) as were the rates of procedural success (BRS vs EES: 88.6% vs 89.9%; P=.854).
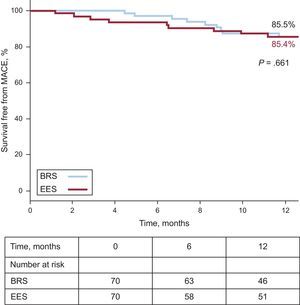
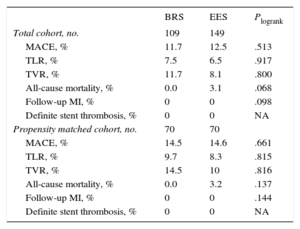
Clinical Outcomes in the Propensity Matched CohortClinical outcomes in the overall overlap cohort are presented in Table 4. Mean follow-up time for patients implanted with BRS was 14.6±6.3 months and 25.3±18 months for EES-treated patients (P<.001). In the propensity matched cohort, at 1-year follow up, the estimated MACE rate (Figure 3) was not significantly different between the 2 groups (BRS vs EES: 14.5% vs 14.6%; Plog-rank=.661). Similarly, no significant differences were seen in 1-year rates of TVR (BRS vs EES: 14.5% vs 10%, Plog-rank=.816), TLR (BRS vs EES: 9.7% vs 8.3%; Plog-rank=.815), follow-up MI (BRS vs EES: 0% vs 0%; Plog-rank=.144) or all-cause mortality (BRS vs EES: 0% vs 3.2%; Plog-rank=.137). No cases of definite stent thrombosis were observed whereas 1 case of probable stent thrombosis occurred in the EES group 40 days after stent implantation (sudden death). Analysis of 1-year TLR for the overlapping lesions in the propensity matched cohort showed no significant differences (BRS: 7.4% vs 7.6%; Plog-rank=.935).
Kaplan Meier Estimated 1-year Outcomes in the Total and the Propensity-matched Cohort
| BRS | EES | Plogrank | |
|---|---|---|---|
| Total cohort, no. | 109 | 149 | |
| MACE, % | 11.7 | 12.5 | .513 |
| TLR, % | 7.5 | 6.5 | .917 |
| TVR, % | 11.7 | 8.1 | .800 |
| All-cause mortality, % | 0.0 | 3.1 | .068 |
| Follow-up MI, % | 0 | 0 | .098 |
| Definite stent thrombosis, % | 0 | 0 | NA |
| Propensity matched cohort, no. | 70 | 70 | |
| MACE, % | 14.5 | 14.6 | .661 |
| TLR, % | 9.7 | 8.3 | .815 |
| TVR, % | 14.5 | 10 | .816 |
| All-cause mortality, % | 0.0 | 3.2 | .137 |
| Follow-up MI, % | 0 | 0 | .144 |
| Definite stent thrombosis, % | 0 | 0 | NA |
BRS, bioresorbable scaffold; EES, everolimus-eluting stent; MACE, major adverse cardiovascular events (death, MI, TVR); MI, myocardial infarction; NA, not available; TLR, target lesion revascularization; TVR, target vessel revascularization.
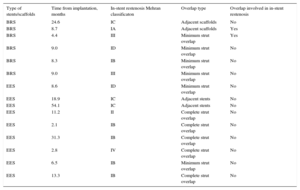
The Mehran classification12 of each restenotic lesion in the 2 groups is presented in Table 5. In patients with angiographic follow-up (BRS group: 6 of 70, EES group: 9 of 70), the overlap was involved in 2 BRS in-stent restenosis patients (Figure 4) but in none of the EES patients (BRS 33.3% vs EES 0%; P=.063), despite the much higher prevalence of complete overlap amongst EES TLR patients (55.5% vs 0%; P=.025).
Characteristics of In-stent/Scaffold Restenosis Involved in Target Lesion Revascularization and Type of Overlap
| Type of stents/scaffolds | Time from implantation, months | In-stent restenosis Mehran classificaton | Overlap type | Overlap involved in in-stent restenosis |
|---|---|---|---|---|
| BRS | 24.6 | IC | Adjacent scaffolds | No |
| BRS | 8.7 | IA | Adjacent scaffolds | Yes |
| BRS | 4.4 | III | Minimum strut overlap | Yes |
| BRS | 9.0 | ID | Minimum strut overlap | No |
| BRS | 8.3 | IB | Minimum strut overlap | No |
| BRS | 9.0 | III | Minimum strut overlap | No |
| EES | 8.6 | ID | Minimum strut overlap | No |
| EES | 18.9 | IC | Adjacent stents | No |
| EES | 54.1 | IC | Adjacent stents | No |
| EES | 11.2 | II | Complete strut overlap | No |
| EES | 2.1 | IB | Complete strut overlap | No |
| EES | 31.3 | IB | Complete strut overlap | No |
| EES | 2.8 | IV | Complete strut overlap | No |
| EES | 6.5 | IB | Minimum strut overlap | No |
| EES | 13.3 | IB | Complete strut overlap | No |
BRS, bioresorbable scaffold; EES, everolimus-eluting stent.
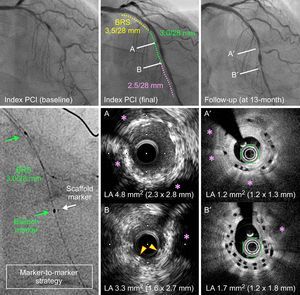
Restenosis at the overlap site shown with optical coherence tomography at the 13-month follow-up angiography of a patient treated with 3 overlapping bioresorbable scaffold (BRS). At 13 months’ follow-up, optical coherence tomography images revealed neointimal proliferation at the site of the overlap leading to a minimum lumen area of 1.7 mm2 (B’). Panels A and B show intravascular ultrasound images (IVUS) at the end of the index procedure. Panels A’ and B’ show optical coherence tomography images at the same level at 13 months’ follow-up. Panels A and A’ show an area of scaffold underexpansion at the index procedure (A), which was characterized by excessive neointimal hyperplasia at follow up (A’). Panels B and B’ show an area of scaffold overlap, which was also underexpanded at the index procedure (B). As a result, at 13-months’ follow-up, neointimal hyperplasia led to in-scaffold restenosis. Pink asterisk: calcified lesions. Yellow arrows show overlapping scaffold struts on initial IVUS (B). LA, lumen area; PCI, percutaneous coronary intervention.
The current propensity matched study demonstrated that stable angina patients with overlapping BRS have comparable outcomes to patients with overlapping EES. Of interest, a third of total TLR occurred at the overlapping site in lesions treated with BRS compared to none for lesions treated with EES, despite the longer overlap segments in the latter. Future, large, purposefully powered randomized controlled studies are required to confirm these findings.
Our results suggest that despite lower acute gain, treatment of coronary lesions with overlapping BRS is feasible with acceptable angiographic and procedural success. A small study from 2 Australian centers19 in 23 patients also suggested that implantation of overlapping BRS was “feasible and safe”, despite the presence of 2 PMI in the overlapping group (8.7%). The very small patient numbers and absence of a control group did not allow the authors of that study to draw any conclusions on the safety and efficacy of overlapping BRS compared with new generation EES. In a larger study (n=1627 patients) from the European multicenter GHOST-EU registry,20 287 (17.6%) patients had lesions treated with overlapping BRS. The patient-oriented clinical endpoint at 1 year was very similar to that reported in the current study (13.6%). Of interest, in the same study, 1-year rates of scaffold thrombosis were similar, yet not negligible, in the overlap vs no overlap group (2.1 vs 2.2%; P=1.000). The 1-year follow-up of the ABSORB-EXTEND study21 (n=812) reported a significantly higher rate of MI amongst patients with overlap vs those without (8.7% vs 2.4%; P=.002), likely reflecting a more complex subset of patients. In our cohort, even though we observed no follow-up MIs, there was a similarly high rate of PMI ∼7.1%, a noteworthy complication. Importantly, the ABSORB-EXTEND also reported a 1.8% rate of scaffold thrombosis in the overlapping BRS group at 1-year, resembling the figure from GHOST-EU. In both GHOST-EU and ABSORB-EXTEND, however, no comparison was made with a new generation EES cohort to provide insights on comparative safety/efficacy.
Even though more than 180000 Absorb BRS have been implanted worldwide, only 6, relatively small, randomized studies have so far been published suggesting comparable outcomes between ABSORB and new generation EES in patients with simple lesions.3–8 The ABSORB II randomized controlled trial,3 despite the lower post implantation acute lumen gain in the BRS group, revealed similar 1-year target lesion failure rates in the 2 groups (BRS vs EES; 5% vs 3%; P=.35). However, since that study was not powered for clinical outcomes, it was followed by the larger ABSORB III4 trial, which included 1322 patients treated with BRS and 686 treated with EES. The occurrence of target lesion failure in the BRS group (7.8%) was noninferior to that in the EES group (6.1%); Pnon-inferiority=.007; Psuperiority=.16. The smaller ABSORB China7 and the ABSORB Japan8 randomized trials similarly revealed no significant differences in 1-year outcomes. The “all-comers” randomized EVERBIO II5 trial (BRS vs EES vs biolimus-eluting stents) and the ABSORB China7 (n=480 patients randomized 1:1 to BRS vs EES) additionally showed no difference in angiographic late lumen loss, at 9 and 12 months, respectively, post stent/scaffold implantation. Furthermore, in the ST-segment elevation myocardial infarction (STEMI) setting, the TROFI II6 trial demonstrated a similar healing response between BRS and EES at 6 months post implantation.
Real-world BRS registries22 have demonstrated overall acceptable mid-term outcomes, albeit with some worrying signs of increased stent thrombosis. Ishibashi et al9 summarized the stent thrombosis experience with BRS in various settings (stable angina, ACS, STEMI). In a total population of 4309 patients implanted with BRS (Absorb v1.1), followed-up for 10.3 months, definite/probable stent thrombosis was seen in 1.22% of patients, of which 0.16% were acute and 0.76% subacute. Stent thrombosis occurred in 0.94% of patients presenting with stable angina, 2.16% in those with ACS, and 1.22% in patients with STEMI. As mentioned above, the 1-year rates of probable/definite scaffold thrombosis in patients treated with overlapping BRS hover around 2%.20,21 These figures raised some concerns when compared with the very low annual definite/probable stent thrombosis rates of 0.89% reported in studies using new generation EES.23,24 These concerns were recently confirmed in a meta-analysis of the 6 randomized trials available to date (n=3738 patients),25 which showed a significantly higher risk of subacute definite/probable stent thrombosis in BRS patients.
The main current drawback of everolimus BRS (Absorb v1.1) relates to its strut thickness and width (157 × 190.5μm for the 2.5mm and 3.0mm scaffolds and 157 × 216μm for the 3.5mm scaffold), which may render it more thrombogenic, particularly when underexpanded or malapposed. In areas of overlap, stacked struts could reach a thickness of ∼300μm. In a porcine model implanted with BRS, in coronary artery segments with complete overlap (ie, with multiple stacked struts) delayed endotheliazation (BRS: 80.1% vs EES 98.2%; P<.001) was observed at 28 days10 compared with the EES group, whereas increased neointimal thickness was seen in the BRS group at 90 days. In real life intervention, adjacent positioning of BRS struts (by careful positioning of the platinum markers so that they are side by side (Figure 1) without overlap can be a very challenging task. Geographical miss (gap between BRS) or complete overlap (either with reduced numbers or multiple numbers of stacked struts) often occurs (Figure 2), potentially predisposing to future TLR. In the current study, a third of in-stent restenosis occurred at the overlap site in patients treated with BRS (BRS 33.3% vs EES 0%; P=.063), concurring with the results of the porcine model mentioned above. Of interest, no overlap in-stent restenosis was observed among EES patients despite the longer overlap segments (Table 5).
LimitationsThe limitations of the current study include the small sample size, the nonrandomized design, and the lack of: a) routine angiographic and intracoronary imaging (IVUS, optical coherence tomography) follow-up, and b) independent core-lab analysis of angiographic findings. Furthermore, despite propensity matching for patient characteristics, there still remained significant differences in baseline lesion characteristics (such as calcific lesions, IVUS use, bifurcation lesions) that could have biased the results.
CONCLUSIONSThis small propensity matched study demonstrated that treating long lesions with overlapping BRS is feasible with acceptable procedural and clinical outcomes. Future, large randomized trials are needed to assess the clinical performance of BRS compared with new generation EES in patients implanted with overlapping BRS.
Conflicts of interestNone declared.
- -
Randomized trials have shown noninferior clinical outcomes in patients with simple lesions treated with bioresorbable eluting scaffolds compared with everolimus eluting stents.
- -
Some concerns, however, have been raised in recent meta-analyses and real-world registries regarding higher rates of scaffold thrombosis with bioresorbable scaffolds.
- -
No study to date has investigated the impact of overlapping scaffolds on angiographic or clinical outcomes.
- -
The current study demonstrated that implantation of overlapping bioresorbable scaffolds is feasible and safe as long as optimal implantation techniques and intracoronary imaging, are used.
- -
Large randomized studies are required to assess in detail the performance and clinical outcomes of overlapping bioresorbable scaffolds when compared with new generation everolimus eluting metallic platforms.