Keywords
INTRODUCTION
Percutaneous treatment of left anterior descending coronary artery (LAD) ostial lesions is complex and remains a focus of research. The therapeutic objective is to guarantee the stent totally covers plaque in the coronary ostium, minimizing possible damage in other bifurcation segments.1-3
Since the introduction of drug-eluting stents (DES)4,5 for left main coronary artery (LMCA) bifurcation, 2 simple strategies seem to have dominated treatment of this lesion type.6 One consists of stent implantation to simultaneously cover the ostial LAD lesion, origin of the circumflex artery (Cx) and, to a greater or lesser extent, distal LMCA. This entails a further planned intervention to adapt the proximal portion of the stent to the bifurcation and the LMCA, usually by the simultaneous inflation of two balloons (the kissing balloon technique).7 The other strategy consists of implanting a stent to cover the LAD ostium with minimal protrusion in the area of the carina.8,9
Pretreatment intravascular ultrasound (IVUS) is considered extremely useful9,10 because it facilitates identification of significant disease in the distal LAD of patients in whom angiography underestimates the degree of stenosis at this level and who therefore require a different percutaneous treatment.
Prior to the introduction of DES, when conventional stents were deployed, the techniques used aimed to diminish plaque volume prior to implantation through directional11 or rotational12 atherectomy, in an attempt to reduce the restenosis rate. However, results tended to be inadequate. The radical reduction of hyperplasia that DES provide has made these techniques redundant except in truly exceptional cases (eg, patients with massive calcification of the lesion).
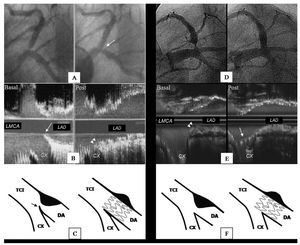
The objective of the present study is to determine the usefulness of an approach to the treatment of LAD ostial lesions that we term the floating-stent technique. It consists of implanting a DES in the proximal LAD to partially cover the origin of the Cx without further planned interventions (Figure 1).
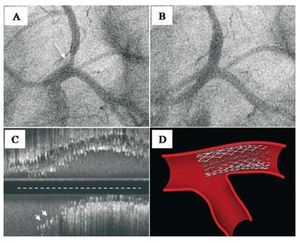
Figure 1. A: LAD ostial lesion (arrow) viewed in LAO-CAU projection. B: angiographic results after floating-stent implantation. C: ultrasound results (longitudinal view) showing stent proximal cells covering Cx origin (arrows). At the level of the contracarina, the stent covers the entire lesion. Note the IVUS catheter trajectory (broken line), out-of-line with respect to the proximal LAD segment axis. D: drawing of floating-stent.
METHODS
Patients
Between February 2002 and September 2008, we treated 71 patients with native LAD ostial lesions (Medina's LMCA classification13 {0,1,0}) by implanting a floating DES to totally cover the LMCA ostium and partially cover the Cx ostium, without needing further planned interventions. Medina's classification assigns a binary value (1,0) depending on whether or not bifurcation segments are affected: LMCA (principal proximal vessel), LAD (principal distal vessel), and Cx (secondary branch). We excluded patients with minimal disease at the level of the distal LMCA and ostial Cx and 21 patients with similar lesions who, during the first 2 years of the study, received a stent covering the distal LMCA and proximal LAD segments. Patient clinical characteristics are in Table 1.
Diagnostic Cardiac Catheterization and IVUS
All patients underwent femoral approach diagnostic catheterization, 30o right anterior oblique ventricular angiography, and multiple sequential coronary angiography projections. The angiographic inclusion criterion was presence of a significant de novo proximal LAD lesion (>50% stenosis) affecting the ostium or the first 3 mm of the ostium. We excluded patients presenting concomitant angiographic LMCA and/or proximal Cx infection. In all patients we calculated tapering14 (quotient obtained by dividing the LAD reference diameter by the LMCA diameter). Baseline angiographic findings are in Table 2.
Forty-nine (69%) patients underwent baseline and post-implantation ultrasound studies (Atlantis SR, Boston Scientific, 2.5 Fr, 40 MHz, or Avanar F/X, Volcano Therapeutics Inc., 2.9 Fr, 20 MHz) with motorized withdrawal at 0.5 mm/s from the distal LAD segment of reference to the LMCA ostium. Nineteen (38%) of these also underwent baseline ultrasound Cx studies. Before withdrawal, 200 µg intracoronary nitroglycerin was administered. We measured external elastic lamina area, luminal area and plaque load at three levels: distal segment of reference, segment of maximum stenosis and distal LMCA. We calculated the remodeling index (external elastic lamina area at the level of the ostium/ external elastic lamina area in the distal segment of reference). Simultaneously, we studied the carinal area in detail (ie, the confluence of distal LMCA, Cx origin, and LAD origin) and analyzed atheromous plaque distribution and carina morphology through a longitudinal reconstruction permitting optimal visualization of the bifurcation. After stent implantation, we analyzed LAD ostium scaffolding characteristics and the degree of stent protrusion in the Cx. Table 3 shows baseline ultrasound findings.
Angiographic and ultrasound characteristics indicate substantial differences in vessel caliber between distal LMCA and proximal LAD (tapering, 0.73 [0.1]). The LAD/Cx angle was 95o (38o). Ultrasound analysis of the pathologic mechanism of stenosis at the level of the LAD ostium revealed predominantly negative remodeling in the lesion (remodeling index, 0.80 [0.21]).
The Floating-Stent Implantation Technique
We implanted the stent using a left anterior oblique projection with caudal angulation, and adapted the double angle to patient anatomy. Stent diameter was chosen as a function of distal LAD reference diameter. In all patients, the stent-deploying balloon proximal radio-opaque marker was positioned proximal to the angiographic carina (LAD/Cx angle) to guarantee complete LAD ostium scaffolding even though this implied some protrusion of the stent at the level of the Cx origin. Contrast images were recorded with the stent in the pre-implantation position. Initial stent inflation pressure was 15 atm. During balloon occlusion, contrast was injected to visualize LMCA and Cx. In patients with ultrasound studies, we also took contrast images of the IVUS transductor when LAD, carina and Cx were visible. We used these to guide subsequent positioning of the stent-deploying catheter balloon proximal marker (Figure 2). We performed post-implantation angiographic and IVUS studies. In most patients, stent implantation was without predilatation. Eleven (15%) of 71 patients underwent short balloon stent post-dilatation at the level of the LAD ostium, respecting the floating-stent segment at the level of the carina. Procedure data are in Table 4.
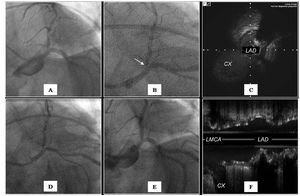
Figure 2.A: LAO-CAU projection angiography showing ostial lesion at the level of the LAD. B: angiography in the same projection showing the IVUS transductor (arrow) at the level of the ultrasound carina. C: transversal ultrasound view showing the carina. D: angiography showing the position of the stent before implantation; the proximal marker of the balloon is positioned with reference to the IVUS transductor. E: angiographic result after stent implantation. F: ultrasound result showing minimal protrusion at the level of the carina and complete covering of the contracarina.
Drug-eluting stents administered sirolimus in 24 (34%) patients, paclitaxel in 11 (15%), everolimus in 25 (35%), and zotarolimus in 11 (15%). During the procedure, patients received 100-200 IU/kg sodium heparin, which was totally or partially neutralized with protamine after the procedure15; they then received 10 000 U of dalteparin/day (10-30 days). All patients received double antiplatelet therapy with aspirin and clopidogrel for ≥1 year.
Angiographic measurements were with CMS 7.1 software (MEDIS, The Netherlands). All angiographic and ultrasound measurements were made off-line by 2 expert interventional cardiologists.
All patients underwent periodic clinical follow-up by telephone or in-clinic.
Statistical Study
Quantitative variables are expressed as mean (SD) and qualitative data as percentages. We compared independent data means with the Student t test and qualitative variables with χ2. We considered P<.05 to be statistically significant. Statistics were calculated with SPSS 15.0.
RESULTS
Angiographic Findings
Immediate procedure success at the level of the LAD was 100%, with minimum 3 (0.42) mm luminal diameter after implantation and 8% (5%) residual stenosis. In no patient did the stent protrude in the LMCA beyond the Cx ostium so post-dilatation was not required at this level.
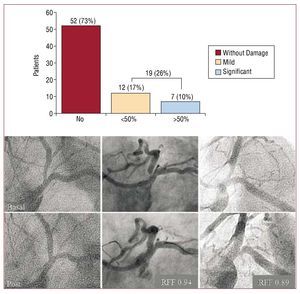
We found focal angiographic disease in the Cx ostium in 19 (27%) patients. This was mild in most cases and significant (residual stenosis >50%) in only 7 (10%). In these patients, the decision to intervene at this level was at the discretion of the operator and consisted of balloon dilatation in 2 (3%) and stent implantation at the level of the Cx ostium in 1 (1%). Five of these 7 patients were studied with a pressure guidewire and all of them registered a >0.85 fractional flow reserve. Figure 3 illustrates this.
Figure 3. Above, a bar chart illustrating incidence of damage in the Cx ostium. Only 10% of cases present >50% residual focal stenosis. Below, baseline angiography (upper row) and post-treatment angiography (lower row) of 3 patients with no damage in the Cx (left), mild Cx ostium disease (center) and significant Cx ostium disease (right). RFF indicates fractional flow reserve.
Ultrasound Findings
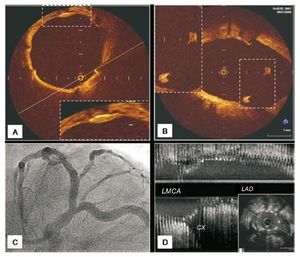
Longitudinal reconstruction IVUS showed absence of plaque at the level of the carina in 36 (73%) of our 49 patients. Ultrasound carina morphology revealed a subgroup of 14 patients (28%) with a spiky carina, reflecting an initially short trajectory parallel to LAD and Cx medial walls and producing a characteristic ultrasound image we have termed the "eyebrow sign" (Figures 4 and 5). In the 49 patients, the stent totally covered the LAD ostium. At the level of the carina we found a protrusion of the stent (2.48 [0.91] mm) covering the Cx origin to a greater or lesser extent. However, opposite the carina, the stent adapted exactly to the LAD ostium and totally covered the plaque at this level without protruding in the LMCA. No patient presented damage at the level of the LMCA.
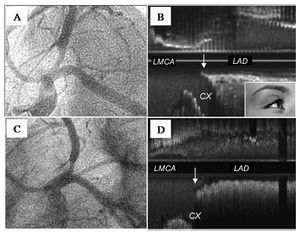
Figure 4.A: angiography of ostial LAD lesion in LAO-CAU projection. B: baseline (longitudinal reconstruction) ultrasound study of the lesion illustrated in A showing, at the level of the carina, the initial parallel trajectory of the medial wall of the LAD and the Cx (arrow); we have termed this peculiar spiky morphology the "eyebrow sign"; note the absence of plaque at the level of the carina. C: another ostial LAD lesion with angiographic characteristics similar to those illustrated in A. D: ultrasound study of the lesion illustrated in C showing the carina without the "eyebrow sign" (arrow). This patient, like most of those in our series, presents no plaque at the level of the carina.
Figure 5. The panel on the left shows a patient with ostial LAD lesion and vulnerable carina anatomy (presence of the "eyebrow sign"). A: baseline and post-treatment angiography; the arrow indicates focal damage induced in the Cx origin after stent implantation. B: baseline and post-treatment ultrasound studies showing the "eyebrow sign" (arrow) and carina displacement (arrow heads). C: drawing illustrating (on the left) lesion with the "eyebrow sign" (arrow); on the right, showing how the stent, protruding in the carinal area, displaces the carina and affects the Cx ostium. The panel on the right shows another patient with favorable carina anatomy. D: baseline and post-treatment angiography showing no Cx ostium disease. E: IVUS examination showing the carina without the "eyebrow sign"; in this patient, note the presence of plaque in the carina (arrow heads); post-treatment, the stent partially covers the Cx origin (arrow) and totally covers the plaque at the level of the contra-carina. F: drawing illustrating the lesion and how the stent adapts to the carinal area without damaging the Cx ostium.
We analyzed angiographic and ultrasound variables that might predict damage at the level of the Cx ostium (Table 5). No significant differences appeared in angiographic parameters between patients with and patients without Cx ostial damage, including LAD-Cx angle amplitude. The only predictor of Cx ostial damage was presence of the "eyebrow sign" in the aforementioned longitudinal IVUS (Figure 5). Of 14 patients who presented this, 13 had disease in the Cx origin after implantation (P<.01).
Figure 6.A, B: optical coherence tomography 7 months post-treatment; pattern of stent endothelization in contact with the wall is similar to that observed when the stent floats in the carinal area. C, D: angiography and ultrasound study of this patient.
Follow-up
The major cardiac event rate was 4% (95% confidence interval [CI], 0.01-1.17). Post-procedure, we recorded non-Q wave infarction (cardiac enzyme elevation more than twice normal) in 2 patients during hospitalization. During follow-up, 1 patient underwent angioplasty for focal restenosis in the LAD. Two patients with clinical recurrence were reevaluated by angiography, which revealed correct positioning of the stent in the LAD but disease progression at other levels. Two patients were reevaluated by catheterization and the stent studied with optical coherence tomography,16 showing a pattern of endothelization in the floating stent cells similar to the portion of the stent in apposition to the LAD wall (Figure 6). In 16 (12) months mean follow-up, we recorded no deaths or late stent thrombosis.
DISCUSSION
Floating-Stent
Percutaneous treatment with DES is an alternative to surgery in symptomatic patients with arteriosclerosis of the LAD ostium.8,9 In the era of the conventional stent, treatment of these lesions was controversial due to the high restenosis rate, which was not reduced by combining stenting with prior resectioning of plaque volume.1,11 With the arrival of the DES, this strategy was abandoned.
Our study shows the usefulness of a simple technique aimed at guaranteeing total covering of the LAD ostium. Although this entails limited protrusion of the stent into the carinal area, ultrasound findings indicate protrusion is limited to the Cx ostium. The positive clinical evolution of our patients suggests the presence of floating cells in the carinal area has no long-term negative impact.
An alternative approach to adjusting stent implantation to the LAD ostium17 consists of advancing the stent over 2 guidewires (LAD and Cx) after inserting the proximal end of a guidewire previously positioned in the Cx through the most proximal stent cell. We consider this complex, not without potential complications, and believe it has not been proven that it guarantees covering the LAD ostium in the contra-carina.
Cubeddu et al7 question strategies of adjusting the stent to the LAD ostium due to the potential risk of not totally covering the lesion. They propose implanting a stent to cover the distal LMCA, Cx origin and proximal LAD lesion followed by further kissing balloon interventions to adapt the stent to the different components of the bifurcation, reporting good results for this approach.
In contrast, the floating-stent technique is simpler and seems to offer good results too. We believe our strategy avoids both incorporating metal in a disease-free segment such as the LMCA, and the need for post-dilatation to achieve correct positioning. Moreover, it avoids the barotrauma this maneuver can produce in the Cx.
The "Eyebrow Sign" as a Predictor of Circumflex Artery Ostial Damage
In LAD ostial lesions, no clear evidence exists of an anatomical configuration unfavorable to stenting. Angiographic information on this seems limited. However, only baseline IVUS can analyze certain specific anatomic details. Longitudinal reconstruction clearly identifies the presence of a spiky carina (the "eyebrow sign"), apparently predicting Cx ostial damage after implantation in the LAD. Stent-induced displacement of this type of carina produced focal indentation in the Cx origin with angiographic patterns that were always similar (Figures 4 and 5). Incidence of this sign in our study is low. However, in patients with this vulnerable anatomy, damage in the side branch ostial could be limited by choosing a stent diameter adjusted to the distal reference of the LAD, thus minimizing carina displacement. When damage occurs in the side branch ostial, as the stent partially covers the Cx origin it permits a guidewire to pass through one of the cells. Low atmosphere balloon dilatation facilitates damage correction through repositioning the carina, but the kissing balloon technique is not employed to avoid barotrauma in the distal LMCA, which is not protected by the stent. In patients in whom this type of carina is very prominent, it may be better to use a different strategy and implant a stent covering the distal LMCA and the proximal portion of the LAD (stent across) to facilitate secondary branch access. We must stress that the "eyebrow sign" is only found in longitudinal reconstruction IVUS, so diagnosis is impossible unless the ultrasound study of the bifurcation shows carina anatomy.
Finally, another ultrasound observation we consider relevant in our series is the documentation of absence of plaque at the level of the carina in most patients (73%). This contradicts previous hypotheses attributing Cx ostial lesions to stent-induced plaque displacement.18,19
Limitations
This descriptive feasibility study lacks a control group. Consequently, we cannot compare the results obtained with those of other techniques. We have no angiographic follow-up of asymptomatic patients treated with the floating-stent technique although, given the localization of the lesion, we believe adverse events are unlikely to occur with no clinical warning. Ideally, our study would include more patients and a longer follow-up.
CONCLUSIONS
Implantation of a floating-stent in the percutaneous treatment of ostial LAD lesions, without disease at the level of the distal LMCA and Cx origin, is a simple technique with a low adverse event rate in the mid-term follow-up. With this technique, the frequency of significant induced damage in the Cx ostium is low (10%). Baseline longitudinal reconstruction IVUS of the bifurcation can identify those patients with consistent vulnerable carina anatomy through the presence of a characteristic pattern (the "eyebrow sign"). This predicts focal angiographic disease in the Cx ostium after floating-stent implantation. The stent appears to displace the carina and induce stenosis. Finally, baseline IVUS shows low incidence of plaque in the carina.
ABBREVIATIONS
Cx: circumflex artery
DES: drug-eluting stent
IVUS: intravascular ultrasound
LAD: left anterior descending coronary artery
LMCA: left main coronary artery
SEE ARTICLE ON PAGES 1221-3
Correspondence: Dr. P. Martín Lorenzo.
Servicio de Cardiología. Hospital Universitario de Gran Canaria Dr. Negrín.
Barranco de la Ballena, s/n. 35010 Las Palmas de Gran Canaria. Las Palmas. España.
E-mail: pemarlor@hotmail.com
Received December 9, 2008.
Accepted for publication June 17, 2009.