Keywords
INTRODUCTION
Left ventricular outflow tract (LVOT) obstruction is a classic feature of hypertrophic cardiomyopathy (HCM) and is observed in 25% of patients.1,2 The obstruction is attributed to functional stenosis in the outflow tract, which is already reduced by septal hypertrophy and aggravated by anterior systolic movement of the mitral valve, and therefore is often accompanied by mitral regurgitation.3,4 The condition is associated with more disabling symptoms, poorer prognosis due to heart failure, and greater risk of death, essentially due to progression of heart failure and stroke.5,6
In most patients, drug therapy can improve the symptoms significantly. Traditionally, therapy is based on the use of beta-blockers and verapamil5-7; however, this should be avoided in the case of severe obstruction. The use of disopyramide in combination with beta-blockers has also proven to reduce the obstruction and improve the symptoms.8 A fair number of patients will continue to have disabling symptoms despite optimized medical therapy, however. Surgical myectomy and septal alcohol ablation are proven to be effective in reducing the gradient and improving patients' symptoms. Nonetheless, the procedures are not free of complications and require careful preparation and high levels of operator experience.4,9,10
A third alternative is the use of pacemakers with sequential pacing, a technique first introduced in the 1970s.11,12 Because it preexcites the right ventricular apex, pacing produces a paradoxical movement of the interventricular septum that leads to less uniform, less effective ventricular contraction, which reduces the LVOT gradient, the anterior movement of the mitral valve, and the grade of mitral regurgitation and even appears to shrink ventricular wall thickness over the long term.6,13 In selected patients with obstructive HCM and very severe symptoms refractory to optimized therapy, pacemakers can be effective and improve both the clinical symptoms and the LVOT gradient.14-16 However, their real benefit has been questioned because pacemakers have a proven placebo effect.17
The paucity of data obtained in clinical practice (because of the limited use of the treatment) and the small number of studies with long follow-up periods prompted us to study the results obtained at 2 hospitals with HCM outpatient clinics (Hospital General Universitario de Alicante and Hospital Universitario Virgen de la Arrixaca de Murcia) and extensive experience in the use of pacemakers in this type of patient. The decrease in LVOT gradient, reduction in left ventricular wall thicknesses, and improvement in functional capacity were assessed.
METHODS
Patients
All patients treated by ventricular pacing at 2 Spanish hospitals with HCM outpatient clinics were included. Patient inclusion was retrospective and ended in May 2007. Common diagnostic and risk stratification protocols were used. The criterion used to diagnose HCM was a left ventricular wall thickness ≥15 mm in the absence of any other cause that could have led to ventricular hypertrophy. Of a total of 627 patients, a pacemaker was implanted for severe symptoms refractory to optimized medical therapy in 72 (11.5%) (27 men and 45 women; age at time of implant, 64.2 [13.7] years). Before the pacemaker was implanted, 46 (63.9%) were in New York Heart Association (NYHA) functional class II/IV. Most patients (68 cases) were in sinus rhythm and 4 had atrial fibrillation or atrial flutter. A total of 6 (8.3%) patients presented grade I atrioventricular block (AVB) and 23 (31.9%), bundle-branch block (Table 1).
Study Protocol
Prior to pacemaker implantation, demographic data and a guided medical history (particularly NYHA functional class and drug therapy) were collected from each patient and a 12-lead ECG, echocardiography, and symptom-limited treadmill test were performed. In the ECG, the rhythm and duration of the baseline PR and QRS intervals were recorded. The following parameters were measured in the echocardiography study: left ventricular (LV) end-diastolic and end-systolic diameters, ejection fraction, interventricular septal and posterior wall thickness, maximal LV thickness, early (E wave) and late (A wave) LV transmitral filling velocities, E/A ratio, and baseline LVOT obstruction (peak gradient). The presence of anterior systolic movement of the anterior mitral valve leaflet, presence and grade of mitral regurgitation, and planimetry measurements of the regurgitation jet were also assessed after provocative maneuvers. In the case of the stress test, the test protocol, duration, METs, and stress response obtained during exertion were recorded.
Following pacemaker implantation, the type of device was recorded, including data on whether the device had a defibrillator function or not. The need for atrioventricular (AV) node ablation was also recorded, along with whether the AV interval had been programmed or not. If programmed, then the number of times and the value of the programmed intervals were also recorded. The programming method used at both hospitals consisted of modifying the AV pacing interval and assessing the appearance of acute changes in the LVOT gradient, as well as the transmitral filling curves. The curve that achieved the largest gradient decrease without excessive shortening of the filling time was chosen. Information was collected from the checkups scheduled at each hospital (usually yearly), which included a new medical history to assess functional class, 12-lead ECG, echocardiogram, and stress test. The study analysis included preimplantation examinations, clinical characteristics, ECG, and stress test upon completion of follow-up. Follow-up included 2 echocardiography studies: one early test (between 6 months and 1 year after pacemaker implantation) and 1 late test (latest available during follow-up). Any complications resulting from pacemaker implantation were recorded, along with the number and cause of death (cardiac or noncardiac) over the entire follow-up period.
Statistical Analysis
The Kolmogorov-Smirnov test was used to determine whether the quantitative variables showed a normal distribution. Quantitative variables are expressed as mean (SD) if they showed a normal distribution, and as median (interquartile range) if not. Discrete variables are expressed as percentages. For comparison between 2 nonpaired quantitative variables, the Student t test was used if the distribution was normal; otherwise, the Mann-Whitney U test was used. For comparisons between 2 paired variables, the Student t test was used for paired variables in the case of normal distribution and the Wilcoxon test if not normal. Discrete variables were compared using the c2 test. To investigate associations with prognosis, logistic regression was performed and the odds ratios (OR) and respective 95% confidence interval (CI) were calculated. A P value less than .05 was considered statistically significant, and SPSS 15.0 was used for the statistical analysis.
RESULTS
The preimplantation peak LVOT gradient was 87 (61.5-115.2) mm Hg and the maximal thickness, 21 (19-24) mm. Regarding therapy, 36 (48.6%) patients were receiving a beta-blocker (BB) and 11 (15.3%) verapamil in monotherapy; 23 (31.9%) patients were receiving combined therapy with a BB + calcium channel blocker (CCB) and 2 (2.7%), BB + disopyramide. Only 1 (1.5%) was receiving triple therapy with a BB, CCB, and disopyramide (Table 1). Of the 72 who received a pacemaker, DDD-R pacing was programmed in 31 (43.1%), DDD mode in 27 (37.5%), VDD mode in 5 (6.9%), and VVI-R mode in 1 (1.4%). Fourteen (19.4%) of all devices had a defibrillator function.
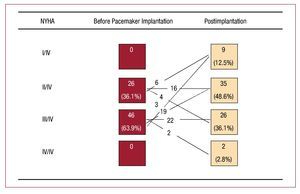
Six patients required AV node ablation to ensure ventricular capture. Following pacemaker implantation, 31 (43.1%) patients presented clinical improvement (Figure). Interestingly, there was a progressive decrease in peak LVOT gradient, such that the preimplantation gradient of 87 (61.5-115.2) mm Hg dropped to 30 (18-54.5) mm Hg in the first echocardiogram and to 17.5 (9.5-47) mm Hg in the last echocardiography study (all, P<.01). Additionally, a reduction was observed in maximal LV wall thickness from 22.1 (4.5) to 19.8 (3.6) mm in the last follow-up and in interventricular septal thickness from 21.8 (4.7) to 19.4 (3.7) mm (both, P=.001) (Table 2).
Figure. NYHA functional class before and after pacemaker implantation. Changes are shown for functional class, comparing pre-pacemaker class, and for patient follow-up (χ2 test, P=.032).
In patients who presented clinical improvement, the reduction in interventricular septal thickness was almost significant in the first echocardiogram (P=.052) and significant in the second echocardiographic follow-up (P=.016). The decrease in maximal LV thickness was also not significant in the first echocardiogram (P=.427) and significant in the second (P=.05). The LVOT gradient decreased significantly in both the first and second echocardiography (P<.001). In patients who did not improve clinically, there were no significant differences in interventricular septal thickness (P=.197), but there were differences in maximal LV thickness (P=.008), although only in the last echocardiographic study performed. In contrast, significant differences were also found in the LVOT gradient reduction (P<.001).
The duration of the stress test and METs did not show a significant change: from 5.2 (2.3) to 5.5 (2.3) min (P=.498) and from 6 (3.3) to 5.1 (2.3) METs (P=.815) before and after pacemaker implantation (Table 2).
Following implantation, 6 (8.3%) patients were able to continue without therapy, 31 (43.1%) received BB in monotherapy, 5 (6.9%) received a CCB, 22 (30.6%) had combined therapy with BB + CCB, 5 (6.9%) had BB + disopyramide, and 3 (4.2%) received triple therapy (BB, CCB, and disopyramide).
When the possible predictors of clinical improvement were analyzed, it was observed that female sex was associated with an improvement in functional class after pacemaker implantation (OR=3.4; 95% CI, 1.22-9.67; P=.020). Women showed a greater improvement (24 of 45, 53%) than men (7 of 27, 26%) (P=.023). In addition, patients in functional class III/IV showed a significant improvement (54.3%) compared with those in functional class II/IV (23.1%) (OR=4.17; 95% CI, 1.42-12.23; P=.009) (Table 3). In the multivariate logistic regression analysis, only a more advanced functional class retained statistical significance (OR=3.12; 95% CI, 1.01-9.77; P=.048).
Of the total of 72 patients, 5 (6.9%) presented pacemaker-related complications: 1 pneumothorax, 1 bacteremia, 2 bacterial endocarditis that required pacemaker withdrawal, and 1 systolic dysfunction that improved once pacing was inhibited. Over the course of the study, 9 patients died (4 from heart failure, 1 sudden death, and another 4 from a noncardiologic cause), 2 patients required myectomy and 2 had septal ablation.
DISCUSSION
We present one of the largest series in Spain in which the possible long-term benefit of using pacemakers for LVOT gradient and the clinical outcomes are analyzed. The present study confirms that the peak LVOT gradient and maximal LV thickness decreased significantly in the long term. In addition, a progressive decrease in both parameters was observed, which confirmed ventricular remodeling secondary to pacing. A clinical improvement was also seen in a high percentage of patients, which confirms the results found by another Spanish group.4 There are, however, no reports in the literature showing that the clinical improvement and the gradient reduction achieved with pacing are important for survival or for reducing major clinical events.18
Although the gradient almost disappeared with medium-term pacing in the patients studied, no other objective evidence of improvement, such as exercise time or METs, was found. Certainly, other pathophysiological mechanisms such as ventricular dysfunction or ischemia can have an influence on the patients' functional deterioration. Tascón et al4 reported a reduction in the grade of mitral regurgitation and the LV filling pressures and improvements in diastolic function and functional class. Previously, Fananazapir et al14 also described significant thinning of the interventricular septum in a patient subgroup. However, as has been shown,19 the natural history of the disease is associated with LV remodeling that leads to a decrease in the estimated left ventricular hypertrophy of 0.6 mm per year. Because our series lacked a control group, an inherent remodeling effect of the disease on thickness reduction cannot be excluded.
Both in the study carried out by Tascón et al4 and in ours, AV pacing does not appear to lead to ventricular function deterioration, as has been suggested in other reports.20 The end-diastolic and end-systolic diameters and the ejection fraction were not significantly different following pacemaker implantation in the patient group. However, we did find that a patient's systolic function worsened with AV pacing and that contractile asynchrony appeared but was corrected when pacing was inhibited. It should be stressed that the AV interval must be adjusted to achieve the optimal value.4,6,7 The ventricular pacing interval should be short enough to ensure early activation of the right ventricular apex without conduction through the native His-Purkinje system, but should also be long enough to permit the contribution made by atrial contraction to ventricular filling. If an excessively short AV interval is selected, then the mean atrial pressure can increase significantly, even if the LVOT gradient is reduced. The optimal interval for each patient should be determined, based on a pacing test in the interventional cardiology laboratory or information obtained by Doppler echocardiography, depending on the hospital's experience with each of these techniques.13 We are guided by the Doppler data and, therefore, program the shortest AV interval that does not reduce the mitral A wave velocity or duration. Systematic AV node ablation is not recommended because the patient would become pacemaker-dependent. In our study, AV node ablation was only performed in 6 (6.2%) patients, always in relation to accelerated AV conduction in the context of atrial fibrillation.
Compared with pacemaker complications (which are uncommon) and the risk of death (which is rare), the risk of surgery is usually high unless it is performed by surgeons who have considerable experience with this condition,21 something not readily available to the entire population. However, the classic nonmedical treatment for the obstruction is surgery. Surgical myectomy performed under the supervision of specialized surgeons clearly improves the symptoms in more than 90% of patients.13 It has been suggested that the LVOT gradient drops below 10 to 20 mm Hg compared to a residual gradient of around 30 mm Hg with ventricular pacing. Long-term follow-up over more than 20 years has shown sustained relief of symptoms in most patients, with no deterioration of systolic function.22 In terms of morbidity and mortality, the septal necrosis caused by alcohol ablation is similar to that of surgery. In addition, a rather common complication of this procedure is AVB.9,10 In this regard, Delgado et al23 recently reported a high prevalence of complete AVB of up to 20%. Septal necrosis by alcohol ablation is associated with a significant risk of complications and perhaps should be reserved for selected patients, especially those who are older and those with comorbidities.
Our data are similar to recent published findings, which report clinical improvement in around 50% of patients but no clear predictor of favorable response to pacing.24 New studies with a larger number of patients and a long clinical follow-up should be carried out to confirm the benefit of pacemakers in patients with severe obstructive HCM refractory to optimized medical therapy. Although advances in cardiac pacing are presently being made, the mechanisms by which electrical pacing in HCM induces changes in cardiac function are not entirely elucidated. Myectomy is certainly the treatment of choice,25 particularly in referral hospitals. In patients who present a high risk for surgery, cannot undergo surgery or septal ablation because of their place of residence, or require permanent pacing for other indications, it may be reasonable to proceed with pacemaker implantation as a first therapeutic alternative to ineffective pharmacological treatment, as indicated in the current guidelines, with a Class IIb recommendation.26
Limitations
In our study, oxygen consumption was not directly measured; hence, the functional capacity of patients had to be indirectly inferred from the duration of exercise and the METs derived from the exercise time and the protocol used. Moreover, the study did not include a control group to compare the course of ventricular gradient and thicknesses.
CONCLUSIONS
In a large series of patients from 2 HCM outpatient clinics, pacemaker implantation reduced the gradient indicating LVOT obstruction and the maximal LV thickness. However, less than half the patients presented subjective improvement in clinical symptoms and only an advanced functional class was shown to be a predictive factor for improvement. Our data confirm the limited role of pacemaker implantation as a treatment for obstruction, but should be considered within the context of the various therapeutic options for such patients.
ABBREVIATIONS
HCM: hypertrophic cardiomyopathy
LVOT: left ventricular outflow tract
NYHA: New York Heart Association
SEE ARTICLE ON PAGES 1217-20
Correspondence: Dr. F. Marín.
Servicio de Cardiología. Hospital Universitario Virgen de la Arrixaca. Ctra. Madrid-Cartagena, s/n. 30120 El Palmar. Murcia. España.
E-mail: fcomarino@hotmail.com
Received September 18, 2008.
Accepted for publication June 17, 2009.