To estimate the health benefits and cost-effectiveness of a polypill intervention (aspirin 100 mg, atorvastatin 20 mg, ramipril 10 mg) compared with multiple monotherapy for secondary prevention of cardiovascular events in adults with a history of myocardial infarction from the perspective of the Spanish National Health System.
MethodsAn adapted version of a recently published Markov model developed and validated in Microsoft Excel was used to compare the cost-effectiveness of the polypill with that of its combined monocomponents over a 10-year time horizon. The population included in the model had a mean age of 64.7 years; most were male and had a history of myocardial infarction. The input parameters were obtained from a systematic literature review examining efficacy, adherence, utilities, and costs. The results of the model are expressed in events avoided, incremental costs, incremental life years, incremental quality-adjusted life years, and the incremental cost-effectiveness ratio.
ResultsOver a 10-year period, use of the cardiovascular polypill instead of its monocomponents simultaneously would avoid 46 nonfatal and 11 fatal cardiovascular events per 1000 patients treated. The polypill would also be a more effective and cheaper strategy. Probabilistic analysis of the base case found a 90.9% probability that the polypill would be a cost-effective strategy compared with multiple monotherapy at a willingness-to-pay of 30 000 euros per quality-adjusted life year.
ConclusionsThe polypill would be a cost-effective strategy for the Spanish National Health System with potential clinical benefits.
Keywords
The recent deterioration in population health and increased prevalence of chronic disease is a worldwide problem with multifactorial and complex causes. Population aging, together with increases in poor nutritional habits, obesity, and hypertension, contribute more and more to the epidemiological development of cardiovascular diseases.1 Accordingly, the population receiving long-term drug therapies and the number of polymedicated patients have significantly increased, exposing the alarmingly low drug adherence rate in both primary and secondary prevention. The situation is so critical that patient adherence to long-term treatments is one of the public health priorities of the European Union.2 Despite the proven efficacy of the drugs used in secondary prevention, the estimated adherence is only 57%.3 This poor therapeutic adherence has an economic and health impact and is associated with the abysmal achievement of therapeutic targets and increased admissions and mortality rates. Both relative and absolute risk estimates show that a considerable proportion of cardiovascular events (about 9% in Europe) can be attributed to poor therapeutic adherence.4 Ho et al.5 found that lack of adherence to cardioprotective drugs was common: 22% for angiotensin-converting enzyme inhibitors (ACEI), 26% for statins, and 29% for beta-blockers. Part of the cost burden of cardiovascular disease springs from a lack of therapeutic effectiveness due to poor adherence. Indeed, the direct and indirect costs of poor adherence in the United States range from 100 000 to 289 000 million dollars per year.6,7 This is one of the factors driving industry, insurance companies, and regulatory and government bodies to identify ways to successfully and cost-effectively promote adherence.
The cost-effectiveness of the polypill has been studied in multiple socioeconomic settings.8 More recently, the results were published of a Markov model created using clinical trial data to analyze the role of a cardiovascular polypill in secondary prevention in the United Kingdom.9 This model compared the use of a polypill (composed of aspirin 100mg, atorvastatin 20mg, and ramipril 10mg) with monotherapy. The model estimated that each 10% increase in adherence could prevent 6.7% of additional fatal and nonfatal cardiovascular events. Based on these and other results, the use of a cardiovascular polypill in secondary prevention could be a strategy with a low cost-effectiveness ratio for preventing cardiovascular events.
In the present article, we compared the cost-effectiveness ratio of a secondary prevention therapeutic strategy involving a polypill containing aspirin 100mg, atorvastatin 20mg, and ramipril 10mg with that of multiple monotherapy in the Spanish taxpayer-funded health system.
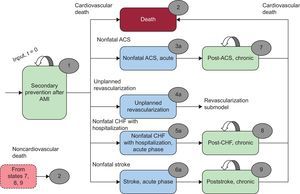
METHODSDesign of the ModelAn adapted version was used of a recently published Markov model9 developed in Microsoft Excel for the United Kingdom with a 3-month cycle length to evaluate cardiovascular outcomes, costs, and benefits and estimate the incremental cost-effectiveness ratio per life year and quality-adjusted life year (QALY) gained from a polypill over a 10-year time horizon. The analysis was performed from the perspective of the Spanish taxpayer-funded health system and included noncardiac mortality data from the Spanish population and costs for Spain. The population included in the model comprised patients older than 40 years who had experienced a myocardial infarction more than 1 year before and who should thus be receiving antiplatelet therapy, preferably with aspirin, a statin, and an ACEI. The population chosen was based on a study by Zeymer et al.10 The population had a mean age of 64.7 years and 72% were men with a previous diagnosis of myocardial infarction. These patients are susceptible to 1 of the following 5 cardiovascular events: acute coronary syndrome, nonfatal stroke, congestive heart failure requiring hospitalization, unplanned revascularization procedures, or death from cardiovascular causes. These patients may also die of noncardiac causes. Patients who have had a nonfatal cardiovascular event remain in the acute phase (health states 3a 4a, 5a, 6a) for 1 cycle of the model; subsequently, they progress to postacute coronary syndrome (health state 7), postcongestive heart failure (health state 8), or poststroke (health state 9). A diagram of the model is shown in Figure 1. The model does not actually reflect all possible options, as that would require the transition probabilities of each state, data currently unavailable in the literature.
Patients could have any of the distinct clinical events according to various key equations reflecting the final probability of a patient experiencing 1 of the 5 cardiovascular events according to patient adherence to the medication. The rates were determined based on adherence to aspirin, statin, and ACEI, as well as the relative risk reduction with each of the 3 medications in specific types of cardiovascular events for adherent and nonadherent patients. A 3% discount rate was applied to the health and cost results.
Input ParametersMost model data were obtained from a literature review largely performed in a previous study.9 That review examined the efficacy of aspirin, statins, and ACEI for secondary prevention in patients with existing cardiovascular disease, costs, health care resource use, utility values associated with existing cardiovascular disease, secondary prevention, and model assumptions. The data on secondary prevention drug adherence in this study were derived from the SPACE collaboration.11
EfficacyThe efficacy of aspirin 100mg, atorvastatin 20mg, and ramipril 10mg for the 5 clinical outcomes was estimated from previous meta-analyses.12⿿15 The reduction in cardiovascular events with aspirin, statin, and ACEI was confirmed, with relative risk reductions of between 0.6 and 0.8. It was assumed that the relative risk reduction with the polypill was similar to that of multiple monotherapy because both approaches involve the same active ingredients and dosages.
CostsThe costs of acute cardiovascular events were obtained from the diagnosis-related groups provided by the Spanish Ministry of Health, Social Services, and Equality16; the costs of the drugs were from the BOTPLUS website.17 The cost of 12.97 euros per month (28 days) of the associated monocomponents was the same as that of the polypill. Other costs were obtained from previous economic models and the literature.18,19 All costs are expressed in euros at 2014 rates.
Probabilities of Events and UtilitiesThe occurrence probabilities of the various cardiovascular events were obtained from placebo groups of meta-analyses16,20 identified in the systematic literature review, which was restricted to the United Kingdom. The probabilities associated with noncardiac death were obtained from life tables for 2012 for men and women in Spain provided by the Spanish National Institute of Statistics.21
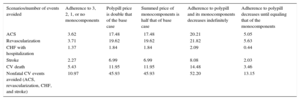
The utility values of the various health states were obtained from different economic modeling studies22⿿24 not specific to Spain (Table 1).
Sources of the Clinical and Economic Parameters of the Model
| Event/conditions/parameter of the model | Baseline probability of events per cycle | Therapeutic efficacy: relative reduction in event risks per individual treatment | Acute event or quarterly cost in chronic phase, euros | Utility values | ||||
|---|---|---|---|---|---|---|---|---|
| Aspirin | ACEI | Statin | Acute | Chronic | Acute | Chronic | ||
| Secondary prevention | NA | NA | NA | NA | NA | 29.5518,a | 0.76022 | 0.83622 |
| CV death | 0.0033914 | 0.8724 | 0.7414 | 0.7514 | 4.20816 | NA | NA | 0.000 |
| Nonfatal ACS | 0.0047414 | 0.7112 | 0.8013 | 0.69 | 3.64718 | 145.5318,b | 0.76022 | 0.83622 |
| Nonfatal stroke | 0.0018514 | 0.7412 | 0.7113 | 0.7214 | 4.95016 | 1043.43c | 0.62922 | 0.69222 |
| Revascularization | 0.0075014 | 0.7112 | 0.8713 | 0.7714 | 10.14516 | NA | 0.78023 | NA |
| Nonfatal CHF with hospitalization | 0.0015420 | 1.00 | 0.8713 | 0.8515 | 3.79816 | 784.8419 | 0.62923 | 0.80024 |
ACEI, angiotensin converting enzime inhibitors; ACS, acute coronary syndrome; CHF, congestive heart failure; CV, cardiovascular; NA, not applicable.
Values express means.
Adherence rates to the polypill and its simultaneously administered monocomponents in the base case were derived from the SPACE trial.11 This collaboration was a meta-analysis of various studies (UMPIRE,25 IMPACT,26 and Kanyini GAP27) reporting the adherence of patients treated with a polypill vs patients treated with the monocomponents given as separate pills. The results showed that, at 15 months, 76% of patients treated with the polypill were adherent vs only 49% of those treated with the separate monocomponents. The proportion of adherent patients was modeled according to an initial maximum value that decreased almost linearly (in reality, exponentially) for 15 months until reaching a fixed and constant value. All adherent patients were assumed to be adherent to the 3 drugs; nonadherent patients were considered to be nonadherent to all drugs.
AnalysisBase CaseThe results are presented as cardiovascular events avoided per 1000 patients, as well as the incremental costs per life year and QALY gained. Due to uncertainty in the literature regarding the persistence of medication adherence, a 10-year time horizon was considered for all evaluated scenarios. In the base case, the adherence percentage was taken from the meta-analysis of the SPACE group11; the monocomponent prices corresponded to the current price in Spain, and a 3% discount rate was applied to costs and benefits. Nonadherent patients obtained no benefit from their treatment and had the same baseline risk of cardiovascular events.
Alternative ScenariosIn these scenarios, the cardiovascular events avoided and economic results were analyzed by varying some of the adherence presumptions, as well as the drug price, both of the polypill and the monocomponents.
The distinct adherence scenarios modeled were as follows: one where the patients could be adherent to 3, 2, 1, or no drugs instead of being adherent to all 3 or none; another where adherence to both treatments decreased indefinitely until 10 years according to the rate of decrease of the base case; and, finally, one where adherence to the polypill decreased until reaching that of the monocomponents, after which the adherence of the 2 types remained constant until 10 years.The prices were modified in 2 scenarios as follows: increasing until reaching double the current polypill price, and decreasing to reach half of the current summed price of the 3 individual monocomponents.
Sensitivity AnalysisTo study the key areas of uncertainty in the model, a deterministic sensitivity analysis was performed of its principal variables. In addition, the stochastic and multivariable uncertainty of the model was evaluated with a probabilistic sensitivity analysis with 10 000 simulations.
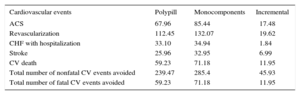
RESULTSThe model revealed that, in the base case and in the 10-year period, 239 and 285 nonfatal cardiovascular events would occur per 1000 patients in the polypill and monocomponents arms, respectively. This would correspond to 46 nonfatal and 11 fatal cardiovascular events avoided with the polypill. The number of patients needed to treat (NNT) with the cardiovascular polypill was 22.2 to avoid a nonfatal cardiovascular event and 45.4 to avoid a fatal cardiovascular event.
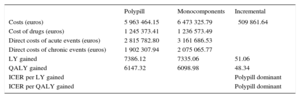
In addition, the economic analysis showed the polypill to be a dominant strategy vs the monocomponents in multiple monotherapy in both incremental costs per life year and QALY gained. The results are shown in Table 2 and Table 3.
Discounted Health Outcomes (Number of Events) for the Base Case (Per 1000 Patients)
| Cardiovascular events | Polypill | Monocomponents | Incremental |
|---|---|---|---|
| ACS | 67.96 | 85.44 | ⿿17.48 |
| Revascularization | 112.45 | 132.07 | ⿿19.62 |
| CHF with hospitalization | 33.10 | 34.94 | ⿿1.84 |
| Stroke | 25.96 | 32.95 | ⿿6.99 |
| CV death | 59.23 | 71.18 | ⿿11.95 |
| Total number of nonfatal CV events avoided | 239.47 | 285.4 | ⿿45.93 |
| Total number of fatal CV events avoided | 59.23 | 71.18 | ⿿11.95 |
ACS, acute coronary syndrome; CHF, congestive heart failure; CV, cardiovascular.
Discounted Economic Outcomes for the Base Case (Per 1000 Patients): Costs and Incremental Costs per Life Year and Quality-adjusted Life Year Gained
| Polypill | Monocomponents | Incremental | |
|---|---|---|---|
| Costs (euros) | 5 963 464.15 | 6 473 325.79 | ⿿509 861.64 |
| Cost of drugs (euros) | 1 245 373.41 | 1 236 573.49 | |
| Direct costs of acute events (euros) | 2 815 782.80 | 3 161 686.53 | |
| Direct costs of chronic events (euros) | 1 902 307.94 | 2 075 065.77 | |
| LY gained | 7386.12 | 7335.06 | 51.06 |
| QALY gained | 6147.32 | 6098.98 | 48.34 |
| ICER per LY gained | ⿿ | ⿿ | Polypill dominant |
| ICER per QALY gained | ⿿ | ⿿ | Polypill dominant |
ICER, incremental cost-effectiveness ratio; LY, life year; QALY, quality-adjusted life year.
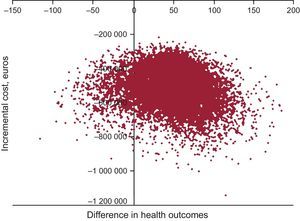
The deterministic sensitivity analysis showed that the incremental cost-effectiveness ratio was more sensitive to the utility value of secondary prevention of chronic myocardial infarction, the utility value of recurrent chronic myocardial infarction, and the discount rate of the benefits (supplementary material). The probabilistic analysis showed that the polypill had a 90.9% probability of being cost-effective with a willingness-to-pay of 30 000 euros per QALY gained in the base case (Figure 2).
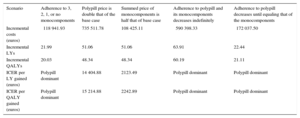
Alternative ScenariosThe polypill was a dominant or cost-effective strategy in all alternative scenarios, reducing both fatal and nonfatal cardiovascular events. The polypill was also cost-effective in the 2 scenarios varying the price of the individual drugs or the polypill itself. In response to the various adherence presumptions, the polypill was a dominant strategy vs the monocomponents, with different effects on cardiovascular events avoided in each scenario. In the first scenario, in which patients could be adherent to 3, 2, 1, or no drugs, 11 nonfatal (NNT = 90.9) and 5 fatal (NNT = 200) cardiovascular events were avoided per 1000 patients treated with the polypill in 10 years. When the adherence to both medication types decreased indefinitely until the end of the model time horizon, 52 nonfatal (NNT = 19.2) and 14 fatal (NNT = 71.4) cardiovascular events were avoided per 1000 patients treated with the polypill in 10 years. Finally, when polypill adherence decreased until it equaled that of the monocomponents, 13 nonfatal (NNT = 76.9) and 3 fatal (NNT = 333.3) cardiovascular events were avoided per 1000 patients treated with the polypill in 10 years. The results obtained in these analyses are detailed in Table 4 and Table 5.
Discounted Health Outcomes in the Alternative Scenarios
| Scenarios/number of events avoided | Adherence to 3, 2, 1, or no monocomponents | Polypill price is double that of the base case | Summed price of monocomponents is half that of base case | Adherence to polypill and its monocomponents decreases indefinitely | Adherence to polypill decreases until equaling that of the monocomponents |
|---|---|---|---|---|---|
| ACS | ⿿3.62 | ⿿17.48 | ⿿17.48 | ⿿20.21 | ⿿5.05 |
| Revascularization | ⿿3.71 | ⿿19.62 | ⿿19.62 | ⿿21.82 | ⿿5.63 |
| CHF with hospitalization | ⿿1.37 | ⿿1.84 | ⿿1.84 | ⿿2.09 | ⿿0.44 |
| Stroke | ⿿2.27 | ⿿6.99 | ⿿6.99 | ⿿8.08 | ⿿2.03 |
| CV death | ⿿5.43 | ⿿11.95 | ⿿11.95 | ⿿14.48 | ⿿3.46 |
| Nonfatal CV events avoided (ACS, revascularization, CHF, and stroke) | ⿿10.97 | ⿿45.93 | ⿿45.93 | ⿿52.20 | ⿿13.15 |
ACS, acute coronary syndrome; CHF, congestive heart failure; CV, cardiovascular.
Discounted Economic Outcomes for the Alternative Scenarios
| Scenario | Adherence to 3, 2, 1, or no monocomponents | Polypill price is double that of the base case | Summed price of monocomponents is half that of base case | Adherence to polypill and its monocomponents decreases indefinitely | Adherence to polypill decreases until equaling that of the monocomponents |
|---|---|---|---|---|---|
| Incremental costs (euros) | ⿿118 941.93 | 735 511.78 | 108 425.11 | ⿿590 398.33 | ⿿172 037.50 |
| Incremental LYs | 21.99 | 51.06 | 51.06 | 63.91 | 22.44 |
| Incremental QALYs | 20.03 | 48.34 | 48.34 | 60.19 | 21.11 |
| ICER per LY gained (euros) | Polypill dominant | 14 404.88 | 2123.49 | Polypill dominant | Polypill dominant |
| ICER per QALY gained (euros) | Polypill dominant | 15 214.88 | 2242.89 | Polypill dominant | Polypill dominant |
ICER, incremental cost-effectiveness ratio; LY, life year; QALY, quality-adjusted life year.
The present study evaluated the potential clinical benefits and cost-effectiveness of a new therapeutic strategy based on the use of a cardiovascular polypill (aspirin 100mg, atorvastatin 20mg, and ramipril 10mg) vs the same monocomponents in separate doses for the treatment of secondary cardiovascular prevention patients from the perspective of the Spanish taxpayer-funded health system. The results show that treatment with the cardiovascular polypill, based on increased therapeutic adherence and, thus, increased real-world effectiveness, could avoid 46 nonfatal and 11 fatal additional cardiovascular events per 1000 patients treated vs the use of the monocomponents given separately. A strategy including the polypill was also a dominant strategy (higher effectiveness and lower cost than the use of the individual components together).
The effectiveness of any therapeutic strategy is determined by the efficacy shown in clinical trials but also by the real-world use (which depends on how many patients are prescribed the approach) in a specific population and the degree of patient adherence to this therapy. In the case of secondary prevention patients considered stable after a coronary event, the efficacy of aspirin, ramipril, and atorvastatin has been widely demonstrated in clinical trials and meta-analyses.13,28,29 Thus, the efficacy of the drugs can be considered to be identical and independent of whether the patient takes these compounds at that dosage in the form of a polypill or as its separate monocomponents. However, although the European guidelines for patients with stable coronary disease recommend with a high level of evidence the use of aspirin, statins, and ACEI in all patients,30 the reality is that these 3 drugs are prescribed together to just 53.3% of patients in Spain,31 with wide variability among the different hospitals in the percentage of patients prescribed these 3 drugs according to the clinical practice guidelines. In addition, analysis of cardiovascular events in patients prescribed aspirin, a statin, and an ACEI together reveals a lower event rate than when these patients are prescribed just 2 of these components.32
An additional problem is that, even when the drugs are prescribed, patient adherence to them remains low, partly due to polymedication.33 A recent study34 showed that the adherence of secondary prevention patients to medication is just 45.5%. These low rates are insufficient to reduce cardiovascular events in real-world situations.35
Given the above premises, the results obtained in our secondary prevention model with the use of a polypill should not be surprising, since the polypill contains the same active ingredients shown to be effective in clinical trials and the approach ensures the homogeneous prescription of aspirin, a statin, and an ACEI to all patients. In addition, as shown by previous studies,11,25⿿27,34,36 the use of a polypill increases treatment adherence while reducing polymedication, helping to ensure that patients are prescribed and take the medication and dosages established by the therapeutic guidelines.
LimitationsSimilar to other studies based on modeling, our study has a series of limitations. In the absence of specific data from the Spanish population, the model was built using certain input parameters obtained from data from other countries. Due to differences in the characteristics of patients in different countries and their health care systems, this could increase the uncertainty of the results. However, the performance of specific sensitivity analyses of these assumptions confirmed the strength of the base case data. In addition, this case has been taken as the basis for considering that adherence is dichotomized into adherence to all 3 monocomponents or to none of them. However, in a more realistic scenario, patients could be adherent to 1, 2, or 3 drugs. Nonetheless, the results of this alternative scenario show that use of the cardiovascular polypill led to a higher number of cardiovascular events avoided vs the use of the monocomponents given separately and would be more effective and cheaper. Another limitation is our consideration that adherence to both treatment types would be constant over time, although other situations might occur in a real-world situation. For example, adherence to both treatment types might decrease indefinitely or adherence to the polypill might decrease until it reaches the same, constant adherence as that of the monocomponents. Nonetheless, in either of these 2 assumptions, the use of the cardiovascular polypill would lead to a higher number of cardiovascular events avoided vs the use of the monocomponents given separately, which is why the polypill is still the dominant strategy.
To sum up, the use of a cardiovascular polypill strategy is dominant compared with the use of monocomponents given separately, both in the proposed base model and in its alternatives, indicating that it is the more effective and cheaper strategy. This is possible due to improved adherence to the polypill and because the price of the polypill in Spain is the same as the summed price of the monocomponents. As an alternative to this price parity scenario, another 2 hypothetical scenarios were developed: 1 in which the price of the polypill was double the current summed price of the components themselves, and another in which the summed price of the monocomponents was half the current polypill price. In both scenarios, the use of the polypill would still be a cost-effective strategy.
CONCLUSIONSIn line with the findings of other groups, the results of the present study show that the use of a therapeutic polypill strategy containing aspirin, atorvastatin, and ramipril compared with the same drugs given separately is cost-effective for secondary cardiovascular prevention, with a potential clinical benefit because the polypill facilitates patient adherence to therapeutic guidelines.
CONFLICTS OF INTERESTV. Barrios, J.M. Castellano, and J. Cosin-Sales have received honoraries for their help in interpreting the results and drafting the manuscript. V. Fuster has no conflict of interest. L. Kaskens, J.E. Ruiz, I. Zsolt, and A. Gracia are employees of Ferrer and helped to perform the study.
- ⿿
Despite the proven effectiveness of the drugs used in secondary prevention, patient adherence is low, estimated to be only 57%.
- ⿿
Patient adherence to long-term therapies is one of the public health priorities of the European Union and a concern for the medical community.
- ⿿
Various studies have shown that polypill-based strategies increase patient adherence and improve health outcomes.
- ⿿
The results of the model studied here show that increased therapeutic adherence due to treatment with a cardiovascular polypill instead of the individual components increases their potential clinical benefit by reducing new event rates.
- ⿿
In addition, the model shows the polypill to be a cost-effective, and even dominant, strategy for numerous scenarios and assumptions, including potential changes in its price and the summed price of the monocomponents, potentially making the polypill the strategy of choice for secondary prevention patients.