We examined whether the rs180070 and rs2070011 polymorphisms of the fibrinogen gene could affect the risk of coronary artery disease in hypertensive patients by modifying the inflammatory process and coagulation.
MethodsA total of 744 participants underwent coronary angiography due to symptoms of stable angina, while hypertension was present in 332 patients.
ResultsThe presence of the A allele (rs180070) was associated with significantly high levels of fibrinogen in hypertensive patients (P=.05). On multivariate analysis, A homozygosity (rs180070) (β = 0.257 ± 18.6; P<.001), but not hypertension status (β = 0.05 ± 11.9; P=.29) was an independent predictor of fibrinogen levels. In hypertensive patients, higher fibrinogen levels>443mg/dL (odds ratio = 3.50; 95% confidence interval, 1.14-10.90; P=.029), but not A homozygosity (odds ratio = 3.00; 95% confidence interval, 0.78-11.90; P = .110) were independent predictors of the presence of coronary artery disease. Moreover, interleukin-6 levels were higher in A homozygotes for the rs180070 polymorphism compared with all other genotypes (P=.046). Indeed, this genotype was the only adjusted independent predictor of interleukin-6 levels (β = 0.151 ± 0.642; P=.032). It was also associated with higher D-dimer levels in hypertension compared with G allele carriers (P=.048).
ConclusionsThe presence of A homozygosity (rs180070) is associated with increased levels of inflammatory mediators and a higher incidence of angiographic coronary artery disease. Importantly, fibrinogen is an independent predictor of the angiographic presence of coronary artery disease in hypertensive patients.
Keywords
The role of inflammatory and thrombotic pathways in atherosclerosis progression is currently well recognized.1,2 Furthermore, hypertension (HT) is major risk factor for cardiovascular disease, while vascular inflammation plays a crucial role in all phases of atherosclerosis. Particularly, in the presence of risk factors such as HT, vascular inflammation is accentuated and associated with clinical manifestations of coronary artery disease (CAD).3,4
Among other biomarkers, fibrinogen is a biomarker with a dual profile. It is involved both in the inflammatory response, thus serving as an inflammatory biomarker, and in coagulation pathways, leading to endothelial dysfunction and atherosclerosis.2,5 In addition, there have been previous reports of an independent association of plasma fibrinogen levels and the risk of CAD.2,5 Patients with acute coronary syndromes generally have higher fibrinogen levels than patients with stable CAD or healthy controls,6 while on some occasions fibrinogen appears to have a greater effect on CAD risk than classical risk factors, such as HT.7
Recent evidence highlights the potential role of fibrinogen genetic variability in the risk of CAD, but the available data are controversial. Therefore, previous studies have suggested potential regulatory effects of genetic polymorphisms, including the rs180070 and rs2070011 polymorphisms on fibrinogen levels and CAD risk, while other studies do not support this possibility.8⿿10 Moreover, the interaction between cardiovascular risk factors and fibrinogen polymorphisms has not been fully elucidated.
In the present study, we aimed to examine the association of fibrinogen single nucleotide polymorphisms with the presence of obstructive CAD in hypertensive patients. In addition, we sought to investigate whether this interaction could result in an increased incidence of angiographic CAD via its effects on the inflammatory process and coagulation.
METHODSStudy PopulationOur study population consisted of 744 participants of Caucasian origin, admitted to our hospital for coronary angiography due to symptoms of stable angina pectoris over a 3-year period. The diagnosis of CAD was established angiographically in the presence of>50% stenosis in at least 1 of the 3 major coronary arteries or major branches. According to the guidelines of the European Society of Cardiology, participants were considered hypertensive if their systolic blood pressure was 140 mmHg and diastolic 90mmHg on 2 different occasions or if they were currently being treated with antihypertensive drugs.11 Participants were considered to have hyperlipidemia if total cholesterol levels 220mg/dL were revealed in biochemical tests or if they were on lipid lowering medication.12 Similarly, participants were considered to have diabetes mellitus if they had fasting plasma glucose levels 126mg/dL, nonfasting plasma glucose levels 200mg/dL, glycosylated hemoglobin A1C>7%, or if they were currently being treated with hypoglycemic agents.13 Current smokers were defined as participants smoking 1 to 10 cigarettes/d for at least the last year.14 Exclusion criteria were the existence of any inflammatory or infective diseases, liver or renal disease, malignancy, heart failure defined as ejection fraction<45%, or a history of deep venous thrombosis or pulmonary embolism. To avoid possible influences of interventional procedures, blood samples were obtained before coronary angiography or percutaneous coronary intervention. Informed consent was obtained from all patients and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
DNA Extraction and GenotypingApproximately 5mL of whole blood was collected in tubes containing ethylenediaminetetraacetic acid. Genomic DNA was extracted from 2 to 5mL of whole blood using standard methods (QIAamp DNA blood kit; Qiagen, West Sussex, United Kingdom). For the detection of both rs180070 (beta chain) and rs2070011 (alpha chain), we used polymerase chain reaction-restriction fragment length polymorphism analysis. To amplify a part of the gene (by polymerase chain reaction) we used the following flanking intronic primers, forward: 5⿲-GAACATTTTACCTTATGTGAATTAAGG-3⿲ and reverse: 5⿲-GAAGCTCCAAGAAACCATCC-3⿲ for the first polymorphism. In addition, the following primers were used for the second polymorphism, forward: 5⿲-TGCAACAGCTTATCGGAAGC-3⿲ and reverse: 5⿲-GTGGAATAAACACCAGAGAG-3⿲. The resulting products were digested by the HaeIII and AciI restriction enzymes respectively (resolution by electrophoresis at 2% agarose gel). Digested fragments were visualized after ethidium bromide staining under ultraviolet light. For polymerase chain reaction quality control, 5% of the samples were randomly selected and genotyped twice for quality assurance, which yielded 100% concordance.
Biochemical MeasurementsAll participants were asked to abstain from smoking and from alcohol- and caffeine- containing beverages during the evening before (12hours) blood sampling. Venous blood samples were centrifuged at 3500rpm at 4°C for 15min, and plasma or serum was collected and stored at ⿿80°C until assayed. Serum levels of fibrinogen were measured by the Clauss method (Multifibren U, Siemens; Germany), D-Dimers by an immunonephelometric method (Innovance D-Dimer, Siemens; Germany), while high-sensitivity C-reactive protein (hsCRP) was also measured. In addition, factor V activity in plasma was measured by a 1-stage assay based on prothrombin time (factor V deficient, Dade Behring; Germany) and factor X activity was measured by a standard 1-stage method using single-donor factor X⿿deficient plasma on a Dade Behring BCS Coagulation Analyzer (Deerfield; Illinois, United States). Interleukin-6 (IL-6) and serum levels of soluble CD40 ligand were measured by ELISA (R&D Systems Inc.; United States).
Statistical AnalysisQualitative variables are presented as relative frequencies. Continuous variables were tested for normal distribution by the Kolmogorov-Smirnov test. Normally distributed data are expressed as mean±standard error of mean. Nonnormally distributed data are presented as the median [interquartile range]. For categorical variables, the chi-square or Fisher exact test was used to compare the distributions for 2 or more groups. Nonpaired student t-tests were used for between-treatment comparisons of continuous variables. We tested whether the allele frequencies conformed to Hardy-Weinberg equilibrium proportions by using the chi-square test. Linkage disequilibrium between the 2 single nucleotide polymorphisms was tested by using the online software of Rodriguez et al.15 Multiple logistic regression analysis was used to estimate odds ratios (OR) and 95% confidence intervals (95%CI) for CAD as a function of the rs180070 and rs2070011 polymorphisms, and fibrinogen levels adjusted for traditional risk factors such as age, sex, HT, diabetes mellitus, hypercholesterolemia, and smoking. Stepwise multivariate analysis was conducted with the measured biochemical parameters as dependent variables and risk factors and polymorphisms as independent variables. Analysis of variance was also used to compare the means of 3 or more variables in the multivariate analysis to determine independent predictors of angiographic CAD and acute myocardial infarction was applied. All significance tests were 2-tailed and conducted at the 5% significance level. All statistical analyses were performed with SPSS (IBM SPSS Statistics, version 22.0).
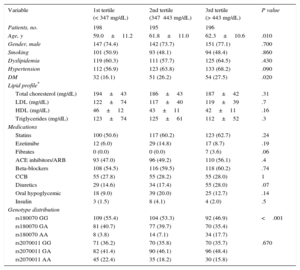
RESULTSThe demographic characteristics of the 744 consecutive patients examined in our center during a 3-year period, as well as the genotype distribution according to fibrinogen tertiles, are presented in Table 1. Of those patients, 332 had HT. Coronary artery disease was present in 448 patients, whereas no CAD was found in 296 patients. Of note, we observed no differences in lipid profile or medication among the 3 groups (Table 1). In our study cohort, the prevalence of the A alleles of rs180070 and rs2070011, respectively, was similar between patients with and without CAD (P=.339 and P=.988, respectively).
Baseline Characteristics According to Tertiles of Fibrinogen Levels
| Variable | 1st tertile (< 347 mg/dL) | 2nd tertile (347⿿443 mg/dL) | 3rd tertile (> 443 mg/dL) | P value |
|---|---|---|---|---|
| Patients, no. | 198 | 195 | 196 | |
| Age, y | 59.0±11.2 | 61.8±11.0 | 62.3±10.6 | .010 |
| Gender, male | 147 (74.4) | 142 (73.7) | 151 (77.1) | .700 |
| Smoking | 101 (50.9) | 93 (48.1) | 94 (48.4) | .860 |
| Dyslipidemia | 119 (60.3) | 111 (57.7) | 125 (64.5) | .430 |
| Hypertension | 112 (56.9) | 123 (63.8) | 133 (68.2) | .090 |
| DM | 32 (16.1) | 51 (26.2) | 54 (27.5) | .020 |
| Lipid profile* | ||||
| Total choresterol (mg/dL) | 194±43 | 186±43 | 187±42 | .31 |
| LDL (mg/dL) | 122±74 | 117±40 | 119±39 | .7 |
| HDL (mg/dL) | 46±12 | 43±11 | 42±11 | .16 |
| Triglycerides (mg/dL) | 123±74 | 125±61 | 112±52 | .3 |
| Medications | ||||
| Statins | 100 (50.6) | 117 (60.2) | 123 (62.7) | .24 |
| Ezetimibe | 12 (6.0) | 29 (14.8) | 17 (8.7) | .19 |
| Fibrates | 0 (0.0) | 0 (0.0) | 7 (3.6) | .06 |
| ACE inhibitors/ARB | 93 (47.0) | 96 (49.2) | 110 (56.1) | .4 |
| Beta-blockers | 108 (54.5) | 116 (59.5) | 118 (60.2) | .74 |
| CCB | 55 (27.8) | 55 (28.2) | 55 (28.0) | 1 |
| Diuretics | 29 (14.6) | 34 (17.4) | 55 (28.0) | .07 |
| Oral hypoglycemic | 18 (9.0) | 39 (20.0) | 25 (12.7) | .14 |
| Insulin | 3 (1.5) | 8 (4.1) | 4 (2.0) | .5 |
| Genotype distribution | ||||
| rs180070 GG | 109 (55.4) | 104 (53.3) | 92 (46.9) | <.001 |
| rs180070 GA | 81 (40.7) | 77 (39.7) | 70 (35.4) | |
| rs180070 AA | 8 (3.8) | 14 (7.1) | 34 (17.7) | |
| rs2070011 GG | 71 (36.2) | 70 (35.8) | 70 (35.7) | .670 |
| rs2070011 GA | 82 (41.4) | 90 (46.1) | 96 (48.4) | |
| rs2070011 AA | 45 (22.4) | 35 (18.2) | 30 (15.8) | |
ARB, angiotensin receptor blockers; ACE, angiotensin-converting enzyme; CCB, calcium channel blockers; DM, diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Unless otherwise indicated, the data are expressed as mean±standard error.
P values based on analysis of variance for continuous normally distributed variables and on the chi-square test for categorical variables.
In the overall cohort, there was a significant effect of the presence of the A allele of rs180070 (AA and AG genotypes) on serum fibrinogen levels (AA or AG: 424.26±7.74 vs GG: 401.20±6.64mg/dL; P=.025). Importantly, the AA homozygotes had significantly higher fibrinogen levels than the G allele carriers (503.4±18.8 vs 402.6±5.14mg/dL; P<.001). In contrast, the presence of the A allele of the rs2070011 allele was not associated with increased fibrinogen levels (AA: 417.1±6.9 vs AG or GG: 404.4±8.8mg/dL; P=.26) in HT patients.
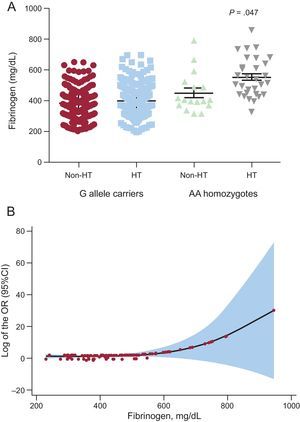
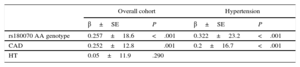
Fibrinogen LevelsPatients were also divided in 3 subcategories according to fibrinogen levels (< 347; 347-443;>443mg/dL) (Table 1). We further examined the effects of these polymorphisms on serum levels of fibrinogen in HT patients. A graded increase of fibrinogen levels was observed: the presence of the A allele of rs180070 (AA and AG genotypes) was associated with significantly higher fibrinogen levels in HT participants (433.36±10.90 vs 405.70±8.8mg/dL; P=.047), while AA homozygosity of rs180070 was also associated with higher fibrinogen levels (541.03±25.20 vs 405.20±6.80mg/dL; P<.001) (Figure 1A). The presence of the A allele of rs2070011 had no significant effects on fibrinogen levels in HT patients. On multivariate analysis, AA homozygosity of rs180070 (β = 0.257 ± 18.6; P<.001) and CAD (β = 0.252 ± 12.8; P=.001) but not HT (β = 0.05 ± 11.9; P=.290) were independent predictors of fibrinogen levels. In HT patients, the AA genotype of rs180070 (β = 0.322 ± 23.2; P<.001) and CAD (β = 0.2 ± 16.7; P<.001) remained independent predictors of fibrinogen levels (Table 2).
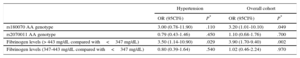
Multivariate logistic regression analysis after adjustment for age, sex, smoking, hypercholesterolemia, HT, diabetes mellitus and log (hsCRP) levels as independent predictors revealed that fibrinogen levels>443mg/dL were associated with a higher risk for CAD (OR=3.90; 95%CI, 1.70-9.40; P=.002) compared with levels<347mg/dL in the general population. In contrast, the intermediate fibrinogen level subcategory had no significant effect on the risk of CAD (OR=1.02; 95%CI, 0.46-2.24; P=.970), while the presence of AA (rs180070) was also significantly associated with an increased risk of CAD (OR=3.20; 95%CI, 1.01-10.10; P=.049) (Table 3).
Effects of Fibrinogen Levels and Fibrinogen Polymorphisms on Coronary Artery Disease Risk
| Hypertension | Overall cohort | |||
|---|---|---|---|---|
| OR (95CI%) | P* | OR (95CI%) | P* | |
| rs180070 AA genotype | 3.00 (0.78-11.90) | .110 | 3.20 (1.01-10.10) | .049 |
| rs2070011 AA genotype | 0.79 (0.43-1.46) | .450 | 1.10 (0.68-1.76) | .700 |
| Fibrinogen levels (> 443 mg/dL compared with<347 mg/dL) | 3.50 (1.14-10.90) | .029 | 3.90 (1.70-9.40) | .002 |
| Fibrinogen levels (347-443 mg/dL compared with<347 mg/dL) | 0.80 (0.39-1.64) | .540 | 1.02 (0.46-2.24) | .970 |
95%CI, 95% confidence interval; OR, odds ratio.
In hypertensive patients, higher fibrinogen levels>443mg/dL (OR=3.50; 95%CI, 1.14-10.90; P=.029) (Figure 1B), but not the AA genotype of rs180070 (OR=3.00; 95%CI, 0.78-11.90; P=.110) were independent predictors of CAD. In our overall cohort, we found that the presence of the A alleles of rs180070 and rs2070011 was not associated with angiographic severity of CAD (OR=1.36; 95%CI, 0.63-2.95; P=.434 and OR=0.99; 95%CI, 0.56-1.78; P=.995 respectively). Finally, neither AA homozygosity of rs180070 nor AA homozygosity of rs2070011 was associated with the occurrence of acute myocardial infarction in the overall cohort (OR=1.87; 95%CI, 0.37-8.90; P=.46 and OR=0.75; 95%CI, 0.39-1.43; P=.378 respectively). Similarly, no association between AA homozygotes (rs180070) and acute myocardial infarction was detected in participants with HT. The unadjusted CAD risk for the carriers of the A allele (rs2070011, AA or AG) was not significant (OR=1.003; 95%CI, 0.72-1.40; P=.988). In the subgroups of patients with HT the unadjusted risk of CAD was not significant for carriers of the A allele rs2070011. Homozygotic status (AA, rs2070011) was not associated with an increased risk for CAD in the overall cohort (OR=1.10; 95%CI, 0.68-1.76; P = .700) (Table 3).
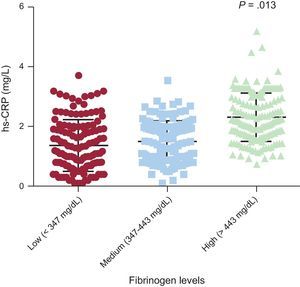
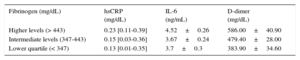
Effects of rs180070 and rs2070011 Polymorphisms on Inflammatory MediatorsIn the general cohort, we found that hsCRP levels were higher in the group of patients with higher fibrinogen levels than in patients with lower and intermediate fibrinogen levels (0.23 [0.11-0.39] vs 0.15 [0.03-0.36] vs 0.13 [0.01-0.35] mg/L; P=.013) (Figure 2). However, AA homozygotes for the rs180070 polymorphism did not have higher hsCRP levels (AA: 0.21 [0.12-0.36] vs AG or GG 0.19 [0.05-0.37] mg/L; P=.6). In addition, in HT patients, hsCRP levels were higher in the group of patients with higher fibrinogen levels than in patients with lower and intermediate fibrinogen levels (0.28 [0.12-0.42] vs 0.17 [0.04-0.38] vs 0.18 [0.09-0.39] mg/L; P=.032).
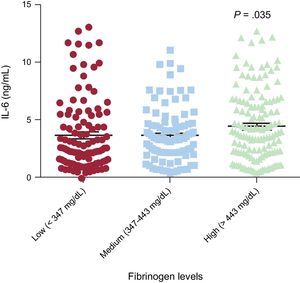
Interleukin-6 levels were higher in the highest subcategory of fibrinogen levels (high fibrinogen levels: 4.52±0.26 vs intermediate fibrinogen levels 3.67±0.24 and vs low fibrinogen levels 3.70±0.30 ng/mL) than in intermediate (P=.027) and low fibrinogen levels (P=.035) (Figure 3). In addition, we found a significant effect of the rs180070 polymorphism on IL-6 levels in HT. Specifically, IL-6 levels were higher in AA homozygotes for the rs180070 polymorphism than in all other genotypes (AG or GG) (4.96±0.69 vs 3.70±0.20 ng/mL; P=.046). In hypertensive patients, multivariate analysis revealed that the AA (rs180070) homozygosity was the only adjusted, independent predictor of IL-6 levels (β = 0.151 ± 0.642; P=.032). On multivariate logistic regression, IL-6 levels were not associated with the risk of CAD, acute myocardial infarction, or multivessel disease in the overall cohort.
The levels of hsCRP were not affected by the presence of homozygotic status for the rs2070011 polymorphism in the overall cohort (P=.486) or in hypertensive patients (P=.677). In the overall cohort, AA homozygosity of rs180070 resulted in significantly higher CD40L levels (2.55±0.35 vs 2.13±0.19 ng/mL; P=.019). However, subgroup analysis of HT patients revealed no differences in CD40L levels in the presence of the AA genotype (rs180070) (P=.144). We observed no significant change in IL-6 or CD40L levels with the presence of the AA genotype of rs2070011 among the studied subgroups.
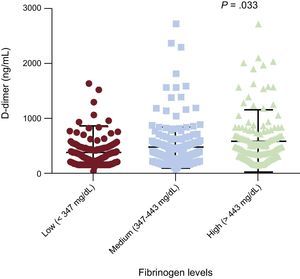
Effects of rs180070 and rs2070011 Polymorphisms on Coagulation FactorsIn the overall cohort, D-dimer levels were higher in the highest subcategory of fibrinogen levels (high fibrinogen levels: 586.00±40.90 vs intermediate fibrinogen levels 479.40±28.00 and vs low fibrinogen levels 383.90±34.60mg/dL) compared with intermediate (P=.03) and low fibrinogen levels (P=.001) (Figure 4 and Table 4). The AA homozygosity of rs180070 resulted in significantly higher levels of D-dimer compared with GG+GA (555.3±43.0 vs 469.1±19.6 ng/mL; P=.033). Similar results were observed for AA homozygosity (rs180070) regarding D-dimer levels in HT compared with the G allele carriers (623.3±79.6 vs 388.6±23.5 ng/mL; P=.048). After adjustment for age, sex, smoking, hypercholesterolemia, HT, diabetes mellitus, and the presence of the AA (rs180070) genotype as independent predictors, multivariate logistic regression analysis revealed that D-dimer levels were associated with CAD (OR=1.001; 95%CI, 1.000-1.002; P=.009). When fibrinogen levels were included in the model, the association between D-dimer and CAD remained significant (OR=1.001; 95%CI, 1.000-1.001; P=.04). In the subgroups of HT patients, logistic regression showed no association between D-dimer levels and CAD. The presence of the rs180070 or rs2070011 polymorphisms was not related to any changes in the levels of factors V, X, plasminogen, and thrombin in HT patients.
Association of Inflammatory Biomarkers With Fibrinogen Levels in the General Cohort
| Fibrinogen (mg/dL) | hsCRP (mg/dL) | IL-6 (ng/mL) | D-dimer (mg/dL) |
|---|---|---|---|
| Higher levels (> 443) | 0.23 [0.11-0.39] | 4.52±0.26 | 586.00±40.90 |
| Intermediate levels (347-443) | 0.15 [0.03-0.36] | 3.67±0.24 | 479.40±28.00 |
| Lower quartile (< 347) | 0.13 [0.01-0.35] | 3.7±0.3 | 383.90±34.60 |
hsCRP, high sensitivity C-reactive protein; IL-6, interleukin-6.
Data are presented as mean±standard error or as median [interquartile range].
To the best of our knowledge, the present study is the first to examine the effects of the rs180070 and the rs2070011 genetic polymorphisms on inflammation, coagulation, and the risk of CAD detected on coronary angiography in HT patients. We observed that AA homozygosity of the rs180070 polymorphism was associated with higher fibrinogen levels and the presence of CAD in the overall cohort, while fibrinogen levels>443mg/dL were independently associated with the presence of CAD in HT. The AA genotype (rs180070) was also associated with higher levels of IL-6 and D-dimer in HT. Similarly, the levels of these parameters were higher in patients with fibrinogen levels>443mg/dL. In the overall cohort as well as in hypertensive patients, hsCRP was found to be elevated in the high-fibrinogen subgroup. These findings indicate that the presence of the AA genotype (rs180070) is associated with increased levels of inflammatory mediators and a higher risk of CAD. However, the most significant predictor of the presence of CAD was elevated fibrinogen levels>443mg/dL. On the other hand, the rs2070011 polymorphism was not associated with the risk of CAD or levels of inflammatory and coagulation mediators.
Hypertension and Fibrinogen Polymorphisms: Risk of Coronary Artery DiseaseThe role of HT in atherosclerosis is well known, while studies have shown that these patients have an increased risk of CAD. In addition, research has been conducted on to the role of fibrinogen polymorphisms in the risk of CAD, with controversial results. Thus, it was reported that plasma fibrinogen gamma⿿ concentration influences the risk of myocardial infarction, possibly due to the positive effect on fibrinogen levels.16 On the other hand, the b-chain fibrinogen gene ⿿148C/T polymorphism showed no association with the risk of CAD.9 More recent evidence showed that there was a significant association between HT and the rs2070008 polymorphism of fibrinogen in women only, whereas fibrinogen haplotypes were not associated with HT after correction for multiple comparisons in either men or women.17 Finally, the rs4220 polymorphism of the fibrinogen beta chain gene was independently associated with plasma fibrinogen level and HT in Chinese participants, suggesting a possible causal role of fibrinogen in HT development, especially in men.18
However, no clear data exist on the role of the studied polymorphisms on the risk of CAD in patients with HT. Our results indicate that elevated fibrinogen levels are an independent predictor of CAD risk in HT participants. In contrast, the rs2070011 polymorphism did not affect fibrinogen levels or the risk of CAD in our cohort. Although the presence of AA homozygosity (rs180070) resulted in higher fibrinogen levels and risk of CAD in the overall cohort, no significant effect was found (in terms of CAD risk) in the subgroup of HT. This finding may suggest that the AA genotype does not raise CAD risk independently of fibrinogen levels and major risk factors such as HT.
Hypertension and Fibrinogen Polymorphisms: Effects on InflammationSeveral studies have shown that HT is associated with increased circulating inflammatory biomarkers.3,4 Although this risk factor has been widely examined, the role of genetics has not been fully clarified in this population. Previously, the fibrinogen beta chain C148T polymorphism was associated with increased fibrinogen, CRP, and IL-6 in patients undergoing coronary artery bypass grafting.19,20 Wong et al.21 reported that there was a clear genetic influence of the IL6-572C>G polymorphism on plasma levels of fibrinogen and CRP, especially in patients with HT. Moreover, the genetic polymorphism A1675G on the AT2R gene affects cardiovascular risk and the severity of the disease by promoting vascular inflammation, particularly in hypertensive men,22 while the M235T polymorphism of the angiotensinogen gene appears to have no effect.23
In the present study, we confirmed that high-risk patients have higher levels of inflammatory biomarkers. In addition, patients with high fibrinogen levels exhibited higher hsCRP, IL-6, and CD40L levels. This finding suggests that among high-risk individuals, concurrent fibrinogen and hsCRP levels may identify those patients with the highest cardiovascular risk. Importantly, we have shown for the first time a significant effect of the rs180070 polymorphism on IL-6 levels among hypertensive patients. Therefore, IL-6 levels were higher in AA homozygotes for rs180070 compared with all other genotypes (AG or GG) and AA homozygosity was the only adjusted independent predictor of IL-6 levels. The inflammatory process was not affected by the presence of homozygotic status (AA) of the rs2070011 polymorphism both in the overall cohort and in patients with HT.
Hypertension and Fibrinogen Polymorphisms: Effects on CoagulationEvidence suggest that increased plasma levels of fibrinogen, D-dimer, and prothrombin fragment 1+2 are present in hypertensive patients with mildly decreased creatinine clearance, suggesting that the coagulation system is activated in these patients.24 However, there are scarce data on the effects of fibrinogen genetic variability on the coagulation cascade of patients with HT. The presence of the Ala379Val polymorphism of lipoprotein-associated phospholipase A2 affected fibrinogen levels in a Caucasian hypertensive population,25 while the fibrinogen alpha Thr312Ala polymorphism was associated with chronic thromboembolic pulmonary HT.26 Furthermore, it has been reported that the rs2227401 and rs2070016 polymorphisms of fibrinogen genes were associated with significantly higher fibrinogen levels,17 whereas the rs1049636 polymorphism was associated with lower concentrations of fibrinogen in women, but not in men.17 Only in a small cohort was it shown that A455 carrier status was associated with increased fibrinogen levels in patients with HT but that study did not examine risk of CAD in this population.27
In our cohort, we found that the presence of the A allele of rs180070 was associated with significantly higher levels of fibrinogen in hypertensive participants. However the presence of the A allele of rs2070011 had no significant effects on the fibrinogen levels in these patients. In the multivariate analysis, the AA genotype of the rs180070 and CAD remained independent predictors of fibrinogen levels. Importantly, we were able to show for the first time that AA homozygosity of the rs180070 polymorphism resulted in significantly higher levels of D-dimer compared with GG+GA and this difference remained significant in patients with HT. Importantly, no differences in lipid profile or medical treatment were observed among the groups, which suggests that initial cardiovascular risk was similar across the fibrinogen tertiles.28 However, the presence of the rs180070 or rs2070011 polymorphisms was not related to any changes in the levels of factors V, X, plasminogen or thrombin in hypertensive patients.
LimitationsThere are some limitations to the interpretation of our data analysis. First, this is a cross-sectional study and we do not report data on clinical follow-up. Additionally, the severity of CAD was not quantified in detail with the use of the SYNTAX score. Finally, potential unmeasured confounders might affect the association between genetic variants and the risk of CAD.
CONCLUSIONSOur study showed that fibrinogen was an independent predictor of the presence of CAD and this strongly related to its levels in HT. Additionally, AA homozygosity of rs180070 was associated with higher fibrinogen, IL-6, and D-dimer levels and risk of CAD in the overall cohort.
CONFLICTS OF INTERESTNone declared.
Special thanks to Ms Magdalini Sgouridi for the technical support.