Late functional tricuspid regurgitation after rheumatic left-sided valve surgery is an important predictor of poor prognosis. This study investigated the usefulness and accuracy of 3-dimensional transthoracic echocardiography tricuspid area compared with conventional 2-dimensional diameter (2DD) for assessing significant tricuspid annulus dilatation, providing cutoff values that could be used in clinical practice to improve patient selection for surgery.
MethodsWe prospectively included 109 patients with rheumatic heart disease in the absence of previous valve replacement. Tricuspid regurgitation was divided into 3 groups: mild, moderate, and severe. Optimal 3-dimensional area (3DA) and 2DD cutoff points for identification of significant tricuspid annulus dilatation were obtained and compared with current guideline thresholds. Predictive factors for 3DA dilatation were also assessed.
ResultsOptimal cutoff points for both absolute and adjusted to body surface area (BSA) tricuspid annulus dilatation were identified (3DA: 10.4 cm2, 6.5 cm2/m2; 2DD: 35 mm, 21 mm/m2); 3DA/BSA had the best diagnostic performance (AUC=0.83). Three-dimensional transthoracic echocardiography tricuspid area helped to reclassify surgical indication in 14% of patients with mild tricuspid regurgitation (95%CI, 1%-15%; P=.03) and 37% with moderate tricuspid regurgitation (95%CI, 22%-37%; P<.0001), whereas 3DA/BSA changed surgery criteria in cases of mild tricuspid regurgitation (17%; 95%CI, 3%-17%; P=.01) compared with 2DD/BSA. On multivariable analysis, right and left atrial volumes and basal right ventricle diameter were independently correlated with 3DA.
ConclusionsThe current 40 mm threshold underestimates tricuspid annulus dilatation. Although 21 mm/m2 seems to be a reasonable criterion, the combination with 3DA assessment improves patient selection for surgery.
Keywords
Functional tricuspid regurgitation (TR) associated with left valvular disease, mainly related to rheumatic etiology, is quite common.1 More than one-third of patients with mitral stenosis have at least moderate TR, and severe TR has been reported in 23% to 37% of patients after mitral valve replacement.2–5 The indication for surgery in TR at the same time as left-sided valvulopathy has moved toward a progressively more interventional attitude in the last few years because the presence of significant TR is closely related to late mortality, cardiac heart failure, and mortality after mitral valve surgery.4,6 Intervention in severe secondary TR is well established as a class I recommendation but is less supported by evidence when functional TR is mild/moderate. In these cases, the presence of significant annulus dilatation (≥ 40 mm or ≥ 21 mm/m2) should be taken into account despite the absence of important TR at baseline (class IIa indication, level of evidence C for European Society of Cardiology and B for the American College of Cardiology guidelines).7,8 These recommendations, together with the frequent difficulty of correct TR evaluation, operator- and interpreter-dependency, and high variability in load conditions, have given rise to assessment of dilatation rather than regurgitation.9–13 Significant tricuspid annulus (TA) dilatation seems to be the underlying mechanism of severe TR but it could also be present in nonsignificant and moderate TR. It is well known that more than one-third of patients with previous nonsevere TR will develop it after surgery; therefore, the identification of significant annulus dilatation in this subgroup of patients is crucial. In patients with functional TR, the TA becomes more circular, planar and dilated in the anteroposterior diameter.14 Thus, TA dilatation might be underestimated with single linear measurements by 2-dimensional (2D) transthoracic echocardiographic (TTE) imaging measured from the apical 4-chamber view. Three-dimensional (3D) TTE offers accurate and real-time assessment of the shape and size of the TA.15–18 Assessment of 3D-TTE tricuspid area (3DA) by 3D-TTE could provide a more objective measurement of TA size; however, to date, no value has been described for this purpose. Thus, the aims of the present study were: a) to explore the influence of clinical and echocardiographic parameters on the severity of functional TR; b) to assess cutoff points of TA dilatation associated with the presence of severe TR, identifying 3DA values that can be used in clinical practice; c) to determine the proportion of nonsevere TR with significant TA dilatation; d) to evaluate whether current guideline criteria properly classify patients for surgery in comparison with 3DA; and finally e) to identify the echocardiographic determinants of TA dilatation with this technology.
METHODSStudy PopulationAn initial sample of 115 consecutive patients referred to our echocardiography laboratory between September 2014 and December 2015 were evaluated with the following inclusion criteria: diagnosis of rheumatic heart valve disease without a history of previous mitral valve replacement, tricuspid valve (TV) repair, or organic TR. During recruitment, 6 patients were excluded due to inadequate echocardiographic images, resulting in a final study population of 109 patients. All patients underwent echocardiography because of clinical indications and gave written informed consent before undergoing echocardiography in accordance with a protocol approved by the institutional review board.
Two-dimensional EchocardiographyTwo-dimensional TTE was performed using a commercially available ultrasound system (iE33; Philips Medical Systems, Andover, Massachusetts, United States) with a fully sampled matrix-array X5 transducer. Two-dimensional diameter (2DD) was measured in the 4-chamber view at the time of the maximum TV diastolic opening between the 2 hinge points of the valvular leaflets. Tricuspid regurgitation degree was graded in 3 groups: mild, moderate, and severe, evaluated by a combination of different echocardiographic parameters according to the recommendations for the echocardiographic assessment of native valvular regurgitation of the American Society of Echocardiography19 including vena contracta width and effective regurgitation orifice area by the proximal isovelocity surface area method. The 3 groups were reassigned in 2 categories: severe and nonsevere TR. Right ventricular diameters (RVD) were measured, adjusting the apical 4-chamber view to acquire the “right ventricular-focused view”. Basal RVD (B-RVD), mid RVD and longitudinal RVD were evaluated.20 Right ventricular systolic pressure (RVSP) was estimated from the TR velocity with the modified Bernoulli equation. Right ventricular (RV) systolic function was assessed by tricuspid annular plane systolic excursion and tissue Doppler-derived tricuspid lateral annular systolic velocity (S’).
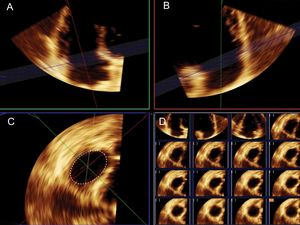
Three-dimensional EchocardiographyUsing the fully sampled matrix-array X5-1 transducer, 3D volumes were obtained with zoomed acquisitions focused on the TV using electrocardiographic gating during a single breath and were digitally stored. In patients with stable sinus rhythm, 4 single-beat volumes were stitched together to create a single volumetric data set. It provides large data sets with high temporal and spatial resolution. The average 3D volume frame rate was 33± 9Hz. Full volume 3D images of the left atrium (LA) and right atrium (RA) were also obtained. For the calculation of left and right atrial volumes, a semiautomated tracing of the endocardial border was performed by marking 3 atrial points. Modifications were made to correct automatic tracings, if necessary. Measurement of 3DA was performed offline using commercial software (QLAB version 9.1; Philips Medical Systems). The 3D volume was sliced and analyzed using multiplanar reconstruction. The orthogonal and cross-sectional planes were adjusted to depict a cross section of the TA as viewed from the RV perspective. After careful orientation, cropping the plane of the TA at its junction with the valvular leaflets, 3DA was calculated by manual tracing at the time of maximum diastolic leaflet opening (Figure 1). Measurements were averaged in 5 cycles in patients with atrial fibrillation.
Measurement of tricuspid annulus area using 3-dimensional transthoracic echocardiography. Sliced 3-dimensional volume. After careful orientation of the annular plane position using 2 orthogonal planes (A and B) the projection of the tricuspid annulus is obtained to measure tricuspid annulus area (C). Right lower panel (D) shows the multislice imaging of the tricuspid annulus.
To assess the effect of observer variability and the reproducibility of 3DA, a second independent observer analyzed 10 randomly selected cases. On the same 3D acquisitions, each observer obtained 3DA, as described above. Intraobserver variability was assessed by comparing the measurements given by the same observer after an interval of more than a week between the 2 measurements. Both readers were blinded to previous measurements.
Statistical AnalysisContinuous variables are presented as mean±standard deviation and were compared using the Student t test. A nonparametric test was used when the data were not normally distributed. Categorical variables are reported as frequencies and were compared by the use of the chi-square and Fisher exact tests. Paired nominal data were compared with the McNemar test. Agreement between techniques was evaluated by the Cohen kappa coefficient. Optimal cutoff value for severe TR detection using both 3DA and 2DD, defined as the maximized value for the sum of sensitivity and specificity, were identified by receiver operating characteristic curves analysis. Interrater reliability was analyzed. Intraobserver and interobserver agreement for 3DA were evaluated by Bland-Altman analysis and intraclass correlation coefficients. Echocardiographic variables (B-RVD, mid RVD, longitudinal RVD, LA volume, RA volume, RVSP, and tricuspid annular plane systolic excursion) were entered in a stepwise multivariable analysis to determine predictors of 3DA dilatation. Statistical significance was defined with P <.05. Statistics were performed using SPSSS version 21 (IBM Inc. Chicago, Illinois, United States) and MedCalc version 10.0.1 (MedCalc Software, Mariakerke, Belgium).
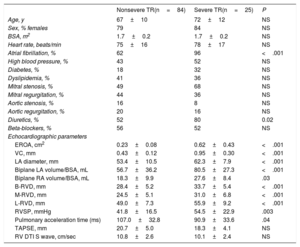
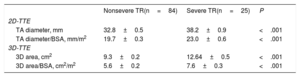
RESULTSInfluence of Clinical and Traditional Echocardiographic Parameters on Functional Tricuspid Regurgitation DevelopmentDemographic and 2D echocardiographic features are shown in Table 1. Severe functional TR was present in 25 patients (23%). Mean age was 68±11 years, 87 patients (80%) were women and 76 (70%) were in atrial fibrillation. There was no difference in age, sex, body surface area, or heart rate between groups. A total of 43 patients (40%) had previous percutaneous mitral valvuloplasty or commissurotomy. The most common left valvular heart disease was mitral stenosis (53%), followed by mitral (42%) and aortic regurgitation (19%), although there was no significant difference with respect to the type of predominant left-sided valvulopathy. The use of diuretics was higher in the group of severe TR. As expected, patients with severe TR had a significantly higher left atrial diameter, 3D left and right atrial volumes, RVSP and RV dimensions. However, no statistically significant differences were found regarding parameters of RV systolic function.
Clinical and 2-dimensional Echocardiographic Features of the Enrolled Participants
| Nonsevere TR(n=84) | Severe TR(n=25) | P | |
|---|---|---|---|
| Age, y | 67±10 | 72±12 | NS |
| Sex, % females | 79 | 84 | NS |
| BSA, m2 | 1.7±0.2 | 1.7±0.2 | NS |
| Heart rate, beats/min | 75±16 | 78±17 | NS |
| Atrial fibrillation, % | 62 | 96 | <.001 |
| High blood pressure, % | 43 | 52 | NS |
| Diabetes, % | 18 | 32 | NS |
| Dyslipidemia, % | 41 | 36 | NS |
| Mitral stenosis, % | 49 | 68 | NS |
| Mitral regurgitation, % | 44 | 36 | NS |
| Aortic stenosis, % | 16 | 8 | NS |
| Aortic regurgitation, % | 20 | 16 | NS |
| Diuretics, % | 52 | 80 | 0.02 |
| Beta-blockers, % | 56 | 52 | NS |
| Echocardiographic parameters | |||
| EROA, cm2 | 0.23±0.08 | 0.62±0.43 | <.001 |
| VC, mm | 0.43±0.12 | 0.95±0.30 | <.001 |
| LA diameter, mm | 53.4±10.5 | 62.3±7.9 | <.001 |
| Biplane LA volume/BSA, mL | 56.7±36.2 | 80.5±27.3 | <.001 |
| Biplane RA volume/BSA, mL | 18.3±9.9 | 27.6±8.4 | .03 |
| B-RVD, mm | 28.4±5.2 | 33.7±5.4 | <.001 |
| M-RVD, mm | 24.5±5.1 | 31.0±6.8 | <.001 |
| L-RVD, mm | 49.0±7.3 | 55.9±9.2 | <.001 |
| RVSP, mmHg | 41.8±16.5 | 54.5±22.9 | .003 |
| Pulmonary acceleration time (ms) | 107.0±32.8 | 90.9±33.6 | .04 |
| TAPSE, mm | 20.7±5.0 | 18.3±4.1 | NS |
| RV DTI S wave, cm/sec | 10.8±2.6 | 10.1±2.4 | NS |
BSA, body surface area; B-RVD, basal RV diameter; DTI, Doppler tissue imaging; EROA, effective regurgitant orifice area; LA, left atrium; L-RVD, longitudinal RV diameter; M-RVD, mid RV diameter; RA, right atrium; RV, right ventricular; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; VC, vena contracta.
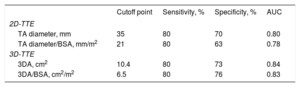
Tricuspid annulus size obtained from 2DD and 3DA following the analysis of 3D-TTE images are summarized in Table 2. Two-dimensional diameter and 3DA were significantly larger in patients with severe TR in both absolute values and those adjusted to body surface area. To evaluate significant TA dilatation (defined as that associated with the presence of severe TR), optimal cutoff points were identified by receiver operating characteristic curves analysis (Table 3). We found that a TA diameter of 35 mm and 21 mm/m2 was the best value to differentiate severe from nonsevere TR groups. For 3DA, a cutoff point of 10.4 cm2 and 6.5 cm2/m2 proved to be just as sensitive as 2DD (80%) but with greater specificity to identify significant TA dilatation associated with severe TR (3DA/BSA: 76% vs 2DD/BSA: 63%).
Tricuspid Annulus Dimensions by 2D and 3D-TTE
| Nonsevere TR(n=84) | Severe TR(n=25) | P | |
|---|---|---|---|
| 2D-TTE | |||
| TA diameter, mm | 32.8±0.5 | 38.2±0.9 | <.001 |
| TA diameter/BSA, mm/m2 | 19.7±0.3 | 23.0±0.6 | <.001 |
| 3D-TTE | |||
| 3D area, cm2 | 9.3±0.2 | 12.64±0.5 | <.001 |
| 3D area/BSA, cm2/m2 | 5.6±0.2 | 7.6±0.3 | <.001 |
2D, 2-dimensional; 3D, 3-dimensional; BSA, body surface area; TA, tricuspid annulus; TR, tricuspid regurgitation; TTE, transthoracic echocardiography.
Sensitivity and Specificity of Echocardiographic Dimensions for Significant Tricuspid Annulus Dilatation Evaluation
| Cutoff point | Sensitivity, % | Specificity, % | AUC | |
|---|---|---|---|---|
| 2D-TTE | ||||
| TA diameter, mm | 35 | 80 | 70 | 0.80 |
| TA diameter/BSA, mm/m2 | 21 | 80 | 63 | 0.78 |
| 3D-TTE | ||||
| 3DA, cm2 | 10.4 | 80 | 73 | 0.84 |
| 3DA/BSA, cm2/m2 | 6.5 | 80 | 76 | 0.83 |
2D, 2-dimensional; 3D, 3-dimensional; 3DA, 3-dimensional area; AUC, area under receiver operating characteristic (ROC) curve; BSA, body surface area; TA, tricuspid annulus; TR, tricuspid regurgitation; TTE, transthoracic echocardiography.
For this purpose, the degree of TR was classified into 3 groups: mild (n=41), moderate (n=43) and severe (n=25). Using a cutoff value of 10.4 cm2, 39% patients with moderate and 15% with mild TR had significant TA dilatation. Also, a nonnegligible percentage of patients, 41% with moderate and 5% with mild TR, had a 3DA ≥ 6.5 cm2/m2, indicative of evident TA enlargement.
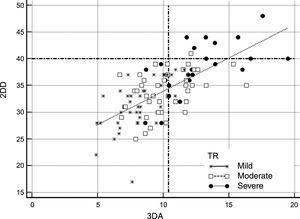
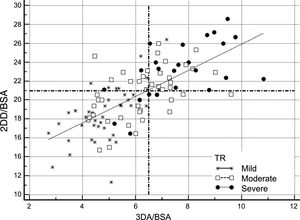
Comparison of 2-dimensional Diameter and 3-dimensional Area Criteria to Select Patients for Tricuspid Valve SurgeryFigure 2 and Figure 3 show potential selection of candidates for surgery based on recommendations of the current guidelines in comparison with the use of 3DA area as a reference parameter for TA dilatation. Figure 2 shows patients who would potentially be referred to TV surgery according to guideline criteria based on absolute 2DD (≥ 40 mm) compared with those who would be selected by using our suggested reference value of 3DA for annular dilatation (≥ 10.4 cm2). Application of the current cutoff value for annuloplasty showed that 9% of patients (95% confidence interval [95%CI], 5%-16%) would have fulfilled absolute 2DD criteria in comparison with 39% (95%CI, 30%-49%) with our absolute 3DA criteria. The estimated difference in percentage was 19.75% (95%CI, 11%-28%; P <.001). This reclassification was evident in all TR degrees. The agreement between the 2 parameters for annuloplasty indication was poor (kappa index: 0.268). Following the classic criteria, no patient with mild TR, only 2.3% with moderate TR, and 36% patients with severe TR would be candidates for surgery. The 3DA would reassign a significant proportion of mild, moderate and severe TR for surgical indication: 14% patients with mild TR (95%CI, 1%-27.5%; P <.001), 37% of moderate (95%CI, 22%-37%; P <.001) and 44% severe TR (95%CI, 19-44%; P <.001). Measurements adjusted by BSA are represented in Figure 3. When the ≥ 21 mm/m2 threshold proposed by the guidelines was considered, differences with respect to the use of 3DA were less conspicuous. Surgical criteria agreement was acceptable (Kappa index: 0.55). The percentage of patients referred to surgery according to the 3DA/BSA changed in only 9% of patients (95%CI,−0.5%-16%; P=.064). This percentage was similar in patients with moderate TR: 9% (95%CI,−8% vs 22%; p=.380) or severe TR: 4% (95%CI,−14%-18%; P=.999). However, significant reclassification was evidenced in patients with mild TR: 17% (95%CI, 3-17%; P=.01). Better specificity of 3DA would exclude from surgery 95% of patients with mild TR in contrast with 78% proposed by 2DD. This might avoid unnecessary interventions in these patients.
Comparison of absolute 2DD and 3DA for selecting candidates for TV surgery according to the guideline threshold of ≥ 40 mm vs ≥ 10.4 cm2. All patients are represented according to the severity of TR in 3 groups (severe: black dots; moderate: white squares; mild: stars). Dashed lines represent the absolute cutoff points proposed by the guidelines (Y axis: 2DD) and the suggested in our work (X axis: 3DA). Following 2-dimensional criteria, most of the patients would be excluded from TV surgery. 2DD, 2-dimensional diameter; 3DA, 3-dimensional area; TR, tricuspid regurgitation; TV, tricuspid valve.
Comparison of 2DD and 3DA (adjusted by BSA) for selecting candidates for TV surgery according to the guideline threshold of ≥ 21 mm/m2 vs ≥ 6.5 cm2/m2. All patients are represented according the severity of TR in 3 groups (severe: black dots; moderate: white squares; mild: stars). Dashed lines represent the absolute cutoff points proposed by the guidelines (Y axis: 2DD/BSA) and those suggested in our work (X axis: 3DA/BSA). Reclassification is evidenced in cases of mild TR. 2DD, 2-dimensional diameter; 3DA, 3-dimensional area; BSA, body surface area; TR, tricuspid regurgitation; TV, tricuspid valve.
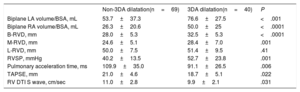
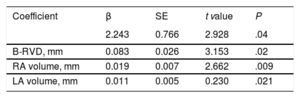
Variables associated with tricuspid 3DA dilatation (≥ 6.5 cm2/m2) in the univariate analysis are presented in Table 4. Stepwise multivariable analysis demonstrated that B-RVD, and left and right atrial volumes were the only echocardiographic independent determinants of TV annular dilatation evaluated with 3D-TTE (Table 5). The B-RVD had statistically the greatest association, with a continuous and direct relationship between B-RVD and 3DA (r: 0.470; P <.001).
Univariable Analysis Results: Determinants of 3DA Dilatation (≥ 6.5cm2/m2)
| Non-3DA dilatation(n=69) | 3DA dilatation(n=40) | P | |
|---|---|---|---|
| Biplane LA volume/BSA, mL | 53.7±37.3 | 76.6±27.5 | <.001 |
| Biplane RA volume/BSA, mL | 26.3±20.6 | 50.0±25 | <.0001 |
| B-RVD, mm | 28.0±5.3 | 32.5±5.3 | <.0001 |
| M-RVD, mm | 24.6±5.1 | 28.4±7.0 | .001 |
| L-RVD, mm | 50.0±7.5 | 51.4±9.5 | .41 |
| RVSP, mmHg | 40.2±13.5 | 52.7±23.8 | .001 |
| Pulmonary acceleration time, ms | 109.9±35.0 | 91.1±26.5 | .006 |
| TAPSE, mm | 21.0±4.6 | 18.7±5.1 | .022 |
| RV DTI S wave, cm/sec | 11.0±2.8 | 9.9±2.1 | .031 |
3DA: 3-dimensional area; BSA, body surface area; B-RVD, basal RV diameter; DTI, Doppler tissue imaging; LA, left atrium; L-RVD, longitudinal RV diameter; M-RVD, mid RV diameter; RA, right atrium; RV, right ventricular; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
Multivariable Analysis: Determinants of 3DA dilatation*
| Coefficient | β | SE | t value | P |
|---|---|---|---|---|
| 2.243 | 0.766 | 2.928 | .04 | |
| B-RVD, mm | 0.083 | 0.026 | 3.153 | .02 |
| RA volume, mm | 0.019 | 0.007 | 2.662 | .009 |
| LA volume, mm | 0.011 | 0.005 | 0.230 | .021 |
3DA, 3-dimensional area; B-RVD, basal right ventricular diameter; LA, left atrium; RA, right atrium; SE, standard error.
Mean intraobserver variability for 3DA measurement was 0.4 cm2 (95%CI,−1.29 to 0.57), whereas mean interobserver variability was 0.7 cm2 (95%CI,−1.88 to 0.52). Intraclass correlation coefficients for intraobserver and interobserver variability were 0.93 (95%CI, 0.84 to 0.99) and 0.87 (95%CI, 0.58 to 0.96), respectively.
DISCUSSIONFunctional TR is an important lesion that leads to worse early and late outcomes after cardiac surgery.21–24 Current society guidelines recommend increased recognition and surgical correction of TR at the time of the concomitant surgery; nevertheless, the presence of secondary tricuspid pathology is often not appreciated, especially if severe TR is not present. Considerable tricuspid dilatation may not always result in pronounced TR and while a general agreement exists for TV repair in cases of severe regurgitation, current guidelines provide more vague indications for patients with less severe TR.7,8 As tricuspid annular dilatation seems to be the underlying mechanism regarding functional TR, it may be a more reliable indicator of TV pathology than TR. Significant dilatation is defined by a diastolic diameter ≥ 40 mm or ≥ 21 mm/m2.20,25 Of note, it has been demonstrated that the annular diameter, measured from the apical 4-chamber view, underestimates major and minor annular dimensions. We propose a new method for assessing tricuspid annular dimension, measuring the maximal diastolic area by 3D-TTE, and its application in the more relevant clinical scenario of functional TR, the rheumatic valve disease. The main results of the present study can be summarized as follows: a) 3DA measurement by TTE is reliable and reproducible; b) severe TR is related to a greater degree of TA expansion, but this enlargement may be present in minor grades of regurgitation but it is poorly identified with diameter measurement; c) 3DA allows a better identification of TA dilatation than 2DD evaluation; d) we defined pathological values of TA area that could be used in clinical practice to consider tricuspid annuloplasty in left-sided rheumatic heart disease; and e) the growth of both atria and the right ventricle are the major determinants of TA area.
Although guidelines and recent data support a proactive approach to surgical repair of TR at the time of mitral valve surgery, TV repair currently appears underused. This attitude comes from an erroneous and historic mistaken selection of patients, more based on the degree of regurgitation than on the evaluation of TA dimension. Since Dreyfus et al.10 notoriously remarked that there is no correlation between TR and tricuspid dilatation (88% of patients in their series with tricuspid dilatation had no or mild TR at preoperative echocardiographic assessment) and the importance of intraoperative TA diameter (> 70 mm) as a criterion for TV repair, the evaluation of the annulus size has been of particular relevance. Because the decision to operate on the TV must be made when surgery is being planned, before patients enter the operating room, it has been proposed to treat, independently of the grade of TR, patients with TA dilatation on 2D echocardiography. However, thresholds for TA dilatation have been obtained from relatively small and heterogeneous patient populations. As can be inferred from our work, based on a larger and homogeneous population, and in agreement with previous literature,9,26 a diameter ≥ 21 mm/m2 seems to correspond to a significant degree of expansion, with a sensitivity of 80%, and a specificity of 63%. Nevertheless, it is important to note that the absolute diameter (regardless of BSA) deviates from the established cutoff points, reinforcing the idea suggested in previous works, in which it is stressed due to the need to better define the 40-mm threshold and it is strongly recommended that it should be lower with increasing degrees of TR and in rheumatic patients.5 In the present study, we found that a value ≥ 35 mm fits more accurately with severe degrees of TR compared with the cutoff of ≥ 40 mm, a very specific value but with a very poor sensitivity (sensitivity of 80% and 70% specificity vs 24% and 99%, respectively).
Therefore, 2 key findings of our study are the necessity to decrease the threshold of TA dilatation to ≥ 35 mm, and the importance of adjusting to BSA. However, we have defined another crucial measurement in the management of these patients: the quantification of the diastolic area by 3D-TTE, which improves the diagnostic accuracy of significant TA dilatation, with greater specificity than 2DD measurement.
In addition, a 3DA ≥ 10.4 cm2 better identified significant TA dilatation in all TR degrees compared with the 40 mm value proposed by the guidelines and, more importantly, allowed reclassification of 14% of patients with mild TR, and 37% with moderate TR. Given that patients with severe TR are candidates for surgery regardless of the size of TA, this subgroup of patients with nonsevere degrees are those who would benefit the most from 3D measurements, especially those with diameters less than 40 mm. It is well known that in patients with less than severe TR, regurgitation might progress after surgery if the TV is untreated. The percentage of nonsevere TR that we could reclassify using 3DA is equivalent to the approximately one-third described by the literature that evolves into late severe TR after surgery follow-up.26,27 Although, according to our results, it seems clear that annular dilatation may be underestimated with single linear measurements, on the other hand, clinicians could wonder if this new measurement may lead to unnecessary interventions in mild degrees of TR. Conversely, this not only did not occur with 3DA estimation, but the better specificity of 3DA/BSA allowed us to reclassify surgical criteria in 17% of mild TR cases (95%CI, 3%-17%; P=.01) in comparison with the proposed ≥ 21 mm/m2 value. This fact might avoid unnecessary surgery in this subgroup of patients. It is important to highlight that the cutoff points obtained in this study are very similar to those recently described by Addetia et al.,28 reinforcing our results. In their recent article, the authors used transthoracic 3D-TTE to characterize TA geometry and dynamics in healthy volunteers, defining the normal tricuspid 3DA as 8.6±2.0 cm2.
Regarding the relationship between 3DA and conventional echocardiographic parameters, the stronger association of RA volume, basal RV and LA volume with TA size found in our study lends support to recent studies conducted with 3D echocardiography, confirming that TA dilatation is an on-going process, which includes the right chambers.14,16,29,30 On the contrary, the absence of an association between RVSP and TA dimensions shows that this phenomenon is not necessarily linked to the presence of pulmonary hypertension, emphasizing the idea that the correction of pulmonary hypertension does not always lead to a decrease in TA size. The absence of significant differences related to RV function between both groups could be related to the limitation of conventional parameters in the presence of preload increase. Probably, the evaluation of other, less preload-dependent parameters (such as ventricular function estimated by deformation parameters) could have shown some discrepancies with these parameters.
The good inter- and intraobserver reproducibility confirms the feasibility and reproducibility of 3D-TTE in assessing TA area, even in the presence of atrial fibrillation.
Finally, this study demonstrates that although 2DD> 21 mm/m2 seems to be a reasonable criterion of marked dilatation of TA in functional TR, the combination with 3DA assessment might improve the selection of candidates for TV surgery. We believe that this approach, based on tricuspid dilatation through the incorporation of this new parameter, and not only on TR degree, allows a more adequate evaluation of secondary TR, a serious and progressive condition that determines the long-term survival of our patients. Future randomized prospective studies are needed to validate these new thresholds in patients who undergo mitral valve surgery.
LimitationsDespite including all degrees of TR, we did not incorporate healthy participants in our work for comparison with patients. Although it would have been of great interest to have normal values, there are recent works that have provided reference values of the TV area by 3D-TTE that support our findings.19 Despite the use of a rigorous method of quantification of the TR, most parameters are influenced by load conditions that can interfere with the correct determination of regurgitation severity. We assessed intra- and interobserver variability of 3DA measurement but not those related to image acquisition, performing all the studies with the same equipment, although acquisition protocols are well standardized and do not substantially differ among vendors. Finally, although we only selected functional TR, excluding cases with organic involvement of the TV, we cannot rule out that there was discrete rheumatic involvement of the TV not visualized by TTE.
CONCLUSIONSTricuspid annulus dilatation is present in a high percentage of patients with nonsevere TR degrees. Although a 2DD cutoff value of 21 mm/m2 seems to be a reasonable criterion, the current 40 mm threshold underestimates real TA dilatation. We provide new parameters with better diagnostic performance related to TA morphology than 2DD, identifying 3DA values ≥ 10.4 cm2 or ≥ 6.5 cm2/m2 as new indicators of significant TA dilatation. This approach might improve the selection of candidates for TV annuloplasty, decreasing the incidence of late TR after rheumatic left-sided valve surgery.
CONFLICTS OF INTERESTNone declared.
- -
It is well known that more than one-third of patients with previous nonsevere TR will develop it lately after surgery; therefore, the identification of significant annulus dilatation in this subgroup of patients is crucial. Tricuspid annulus dilatation might be underestimated with single linear measurements by 2D-TTE.
- -
Tricuspid annulus dilatation is present in a high percentage of patients with nonsevere TR degrees. The main finding of the present study is that current 40 mm threshold underestimates TA dilatation. We provide new 3D parameters with better diagnostic performance. This approach might improve the selection of candidates for TV annuloplasty, decreasing the incidence of late TR after rheumatic left-sided valve surgery.
Statistical analysis assistance was provided by Ramon Mahía, PhD in Econometrics and Quantitative Analysis, permanent professor in the Econometrics and Informatics research unit of the Applied Economy Department at Universidad Autónoma de Madrid, Madrid, Spain.