In everyday practice, clinicians are faced with questions on the comparative benefits and risks of the various health interventions available to treat patients. When performed well and reported completely, randomized controlled trials (and systematic reviews of such trials) are generally considered to be the most scientifically rigorous approach to determine whether a cause-effect relationship exists between an intervention and a given outcome. However, clinical trials are hampered by their tendency to focus on simultaneous comparison of no more than 2 or 3 alternatives (eg, treatment vs no treatment, treatment with option A vs B and A vs C). To make more informed decisions, knowledge of the existing evidence on the numerous alternatives available in clinical practice, as well as quantitative recapitulation of this information, is often required to estimate the comparative efficacy and safety of health interventions. Ideally, approach would require clinical trials with as many treatment arms as there are available alternatives, allowing diverse comparisons to be made between option A and B, B and C, C and D, and so forth, and not only the determination of whether A is simply better (or not worse) than B. This information would also be useful for guiding clinical decision making through the use of systematic reviews and clinical practice guidelines that stringently evaluate the evidence and clearly state which are the best choices for treating a specific patient. However, the information required is often only partially available and/or has major associated uncertainties.
Limited resources and the need to determine in relative terms the beneficial or potentially harmful effects of multiple treatments have meant that, consciously or not, the transitive property is always present in clinical reasoning when choosing a treatment, in the planning and design of a new clinical trial, or, more recently, in the development of new methods of evidence synthesis that consider indirect comparisons, such as network meta-analyses.1–3 In this editorial, the concept of transitivity is presented and illustrated through examples from published clinical trials that examined the effect of different antihypertensive therapies on cardiovascular prevention in high-risk patients.
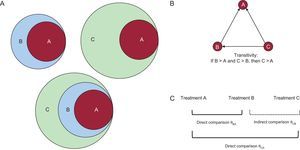
THE CONCEPT OF TRANSITIVITYEssentially, a binary relationship relating to a group of elements is considered to be transitive if it holds that “if A implies B, and B implies C, then A implies C”.4 This relationship can be graphically presented through diagrams in which A is found within B and B is found within C (Figure 1A). Accordingly, the extension of this logical reasoning leads to the argument that, if in one clinical study treatment B is better than treatment A and in another study treatment C is better than B, C can be concluded to be better than A (Figure 2).5–8 However, is this always true?
Transitive property. A: A case in which the transitive property holds, that is, that element A implies B (B > A), A implies C (C > A), C implies B (C > B). B: Triad network (closed loop), in which each node is a treatment and each line indicates the existence of clinical trials that compare each treatment directly with each other (direct comparison); the transitive property holds when treatment B is better than A, treatment C is better than B, and treatment C is better than A. C: 2 direct comparisons (B vs common comparator A and C vs common comparator A); assuming that the transitive property is fulfilled, the effect of treatment C vs A can be indirectly estimated.
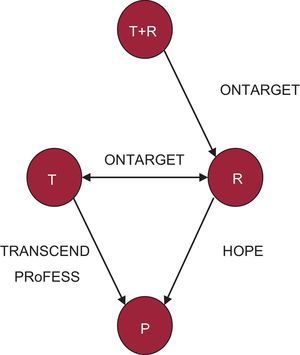
Network of direct comparisons, composed of the HOPE, ONTARGET, TRANSCEND, and PRoFESS trials,5–8 in which each node is a treatment and each line indicates the existence of studies that directly compare each treatment with each other (direct comparison). P, placebo; R, ramipril; T, telmisartan; T+R, combination of telmisartan and ramipril.
Generally, one speaks of a direct comparison when 2 treatments under evaluation have been compared with each other (eg, in a clinical trial or meta-analysis) and of an indirect comparison when 2 treatments have not been directly compared but have been compared head to head in a study, but rather have been assessed via a common comparator intervention. Thus, transitivity can be expressed in a simple manner, with an indirect comparison between treatments correctly estimating an direct comparison that has not been observed. Although transitivity cannot be statistically proven, its plausibility can be conceptually assessed from a more clinical or epidemiological perspective when there are direct comparisons between different treatments that make a closed loop in a network of clinical trials (Figure 1B).3 When direct comparisons are unavailable, one of the established hypotheses when trying to calculate the effect of C versus B involves the use of knowledge of C versus B through a common comparator A (Figure 1C). This use of assumed transitivity allows determination of whether the common comparator A allows a valid comparison to be made between treatments. However, this is an observational approach. In fact, although controlled and randomized clinical trials are used to make comparisons (direct or indirect), the choice of comparisons in each study is not random.
FACTORS THAT CAN AFFECT THE TRANSITIVE PROPERTYIn clinical epidemiology, the term interaction is usually used to describe a situation in which 2 or more factors modify the effect that each situation has on the occurrence or magnitude of a given outcome.9 This phenomenon is also known as effect modification.
For the transitivity assumption to hold, there must be no differences in the distribution of effect-modifying factors among the studies considered. For example, the transitivity assumption may be violated when “old” treatments are compared with more recent alternative therapies because unobserved variables could differ among the comparisons (eg, study quality, changes in the concomitant used over time to manage patients, alterations in the severity of the treated populations due to changes in diagnostic criteria or clinical practice).3 A summary with examples of possible effect-modifying factors in clinical trials is shown in Table 1, distinguishing among aspects related to the patient (population), the intervention, the comparator (or the alternative), and the outcome (the event or disease that one wants to prevent or treat).10 Clinicians and researchers can assess transitivity by carefully reviewing the methodologic properties of the studies and the clinical characteristics of the patient populations.
Summary of Possible Effect-modifying Factors
| Levels | Possible effect-modifying factors |
| Target patient or population | • Age• Sex• Severity (baseline risk)• Disease duration• Comorbidity/comorbidities• No response to previous treatment• Provider or setting* |
| Intervention and comparator | • Type of intervention (eg, preventative, therapeutic)• Dose or intensity• Duration• Route of administration• Concomitant treatments• Complexity• Provider or setting* |
| Outcome | • Outcome definition• Measuring or monitoring tools• Methods and procedures• Follow-up duration |
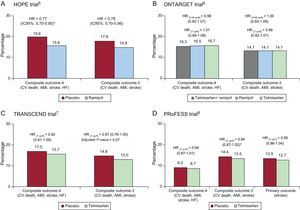
During the last decade, various clinical trials have examined the effect of suppression of the renin-angiotensin system through angiotensin II blockade with angiotensin-converting enzyme inhibitors (ACEIs) or selective blockade of the AT1 receptor with angiotensin II receptor blockers (ARBs) on reducing cardiovascular morbidity and mortality, going beyond their impact on blood pressure. For example, the ONTARGET6 trial, which examined the possible benefits of dual blockade with ACEI and ARB in more than 25 000 patients (Table 2), determined that an ARB (telmisartan) was as effective as an ACEI (ramipril) in reducing cardiovascular morbidity and mortality in high-risk patients. Specifically, the 4.7-year follow-up results showed noninferiority between the ARB and the ACEI in the primary composite outcome of cardiovascular death, acute myocardial infarction, stroke, or hospitalization for heart failure (hazard ratio [HR] = 1.01; 95% confidence interval [95%CI], 0.94-1.09; P = .83). In the same study, combining ACEI and ARB medications failed to offer additional benefits compared with ACEI monotherapy (HR = 0.99; 95%CI, 0.92-1.07; P = .38).
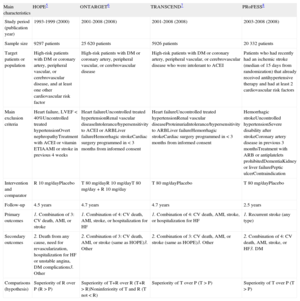
Design of the HOPE, ONTARGET, TRANSCEND, and PRoFESS Trials.5–8
| Main characteristics | HOPE5 | ONTARGET6 | TRANSCEND7 | PRoFESS8 |
| Study period (publication year) | 1993-1999 (2000) | 2001-2008 (2008) | 2001-2008 (2008) | 2003-2008 (2008) |
| Sample size | 9297 patients | 25 620 patients | 5926 patients | 20 332 patients |
| Target patients or population | High-risk patients with DM or coronary artery, peripheral vascular, or cerebrovascular disease, and at least one other cardiovascular risk factor | High-risk patients with DM or coronary artery, peripheral vascular, or cerebrovascular disease | High-risk patients with DM or coronary artery, peripheral vascular, or cerebrovascular disease who were intolerant to ACEI | Patients who had recently had an ischemic stroke (median of 15 days from randomization) that already received antihypertensive therapy and had at least 2 cardiovascular risk factors |
| Main exclusion criteria | Heart failure, LVEF < 40%Uncontrolled treated hypertensionOvert nephropathyTreatment with ACEI or vitamin ETIAAMI or stroke in previous 4 weeks | Heart failureUncontrolled treated hypertensionRenal vascular diseaseIntolerance/hypersensitivity to ACEI or ARBLiver failureHemorrhagic strokeCardiac surgery programmed in < 3 months from informed consent | Heart failureUncontrolled treated hypertensionRenal vascular diseaseProteinuriaIntolerance/hypersensitivity to ARBLiver failureHemorrhagic strokeCardiac surgery programmed in < 3 months from informed consent | Hemorrhagic strokeUncontrolled hypertensionSevere disability after strokeCoronary artery disease in previous 3 monthsTreatment with ARB or antiplatelets prohibitedDementiaKidney or liver failurePeptic ulcerContraindication |
| Intervention and comparator | R 10 mg/dayPlacebo | T 80 mg/dayR 10 mg/dayT 80 mg/day + R 10 mg/day | T 80 mg/dayPlacebo | T 80 mg/dayPlacebo |
| Follow-up | 4.5 years | 4.7 years | 4.7 years | 2.5 years |
| Primary outcomes | 1. Combination of 3: CV death, AMI, or stroke | 1. Combination of 4: CV death, AMI, stroke, or hospitalization for HF | 1. Combination of 4: CV death, AMI, stroke, or hospitalization for HF | 1. Recurrent stroke (any type) |
| Secondary outcomes | 2. Death from any cause, need for revascularization, hospitalization for HF or unstable angina, DM complications3. Other | 2. Combination of 3: CV death, AMI, or stroke (same as HOPE)3. Other | 2. Combination of 3: CV death, AMI, or stroke (same as HOPE)3. Other | 2. Combination of 4: CV death, AMI, stroke, or HF3. DM |
| Comparisons (hypothesis) | Superiority of R over P (R > P) | Superiority of T+R over R (T+R > R)Noninferiority of T and R (T not < R) | Superiority of T over P (T > P) | Superiority of T over P (T > P) |
ACEI, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blockers; CV, cardiovascular; DM, diabetes mellitus; HF, heart failure; LVEF, left ventricular ejection fraction; P, placebo; R, ramipril; T, telmisartan; TIA, transient ischemic attack.
Evaluation of the effect of the ARB telmisartan was performed in 2 other studies: TRANSCEND7 and PRoFESS.8 The TRANSCEND trial compared the effect of an ARB and a placebo in reducing cardiovascular morbidity and mortality in almost 6000 high-risk patients with diabetes, end-organ damage or coronary artery, peripheral vascular, or cerebrovascular disease, with history of ACEI intolerance but without albuminuria or heart failure (Table 2). In the study, after a 4.7-year follow-up, no significant effect was seen with an ARB (telmisartan) vs placebo for the primary composite outcome of cardiovascular death, acute myocardial infarction, stroke, or hospitalization for heart failure (HR = 0.92; 95%CI, 0.81-1.05; P = .22). The secondary outcome, which omitted hospitalization for heart failure, also failed to show a clear beneficial effect (HR = 0.87; 95CI%, 0.76-1.00; P = .07, adjusted for multiplicity). Placebo-treated patients received significantly more diuretics (40% vs 34%; P < .0001) and calcium antagonists (46% vs 38%; P < .0001) than those treated with ARB.7 The PRoFESS trial, performed in more than 20 000 patients, compared the ARB telmisartan with placebo to assess the reduction in stroke recurrence (Table 2). After a 2.5-year follow-up, no significant differences were seen between the groups for the primary outcome (HR = 0.95; 95%CI, 0.86-1.04; P = .23), in the secondary outcome of cardiovascular death, acute myocardial infarction, stroke, and heart failure (HR = 0.94; 95%CI, 0.87-1.01; P = .11), or in the tertiary outcome of cardiovascular death, acute myocardial infarction, or stroke (HR = 0.94; 95%CI, 0.87-1.02; P = .13).
Notably, some of these data appear to contradict or disagree with those of previous studies, such as those of the HOPE5 trial, which showed a clear benefit of antihypertensive treatment with ACEI in reducing cardiovascular events (see the network diagram in Figure 2). Specifically, the HOPE trial demonstrated the benefit of renin-angiotensin system blockade through an ACEI (ramipril) vs placebo in high-risk patients with diabetes or coronary artery, peripheral vascular, or cerebrovascular disease, and at least 1 other cardiovascular risk factor. Follow-up results at more than 4 years showed the superiority of an ACEI (ramipril) over placebo in the primary composite outcome of cardiovascular death, acute myocardial infarction, and stroke (HR = 0.78; 95%CI, 0.70-0.86; P < .001).
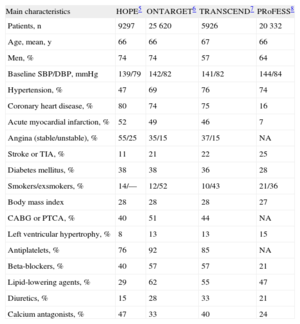
The study and patient characteristics in each of the above trials are summarized in Tables 2 and 3. The main results of the different trials are summarized in Figure 3.5–8,11
Baseline Characteristics of the Patients and Treatments in the Example Trials
| Main characteristics | HOPE5 | ONTARGET6 | TRANSCEND7 | PRoFESS8 |
| Patients, n | 9297 | 25 620 | 5926 | 20 332 |
| Age, mean, y | 66 | 66 | 67 | 66 |
| Men, % | 74 | 74 | 57 | 64 |
| Baseline SBP/DBP, mmHg | 139/79 | 142/82 | 141/82 | 144/84 |
| Hypertension, % | 47 | 69 | 76 | 74 |
| Coronary heart disease, % | 80 | 74 | 75 | 16 |
| Acute myocardial infarction, % | 52 | 49 | 46 | 7 |
| Angina (stable/unstable), % | 55/25 | 35/15 | 37/15 | NA |
| Stroke or TIA, % | 11 | 21 | 22 | 25 |
| Diabetes mellitus, % | 38 | 38 | 36 | 28 |
| Smokers/exsmokers, % | 14/— | 12/52 | 10/43 | 21/36 |
| Body mass index | 28 | 28 | 28 | 27 |
| CABG or PTCA, % | 40 | 51 | 44 | NA |
| Left ventricular hypertrophy, % | 8 | 13 | 13 | 15 |
| Antiplatelets, % | 76 | 92 | 85 | NA |
| Beta-blockers, % | 40 | 57 | 57 | 21 |
| Lipid-lowering agents, % | 29 | 62 | 55 | 47 |
| Diuretics, % | 15 | 28 | 33 | 21 |
| Calcium antagonists, % | 47 | 33 | 40 | 24 |
CABG, coronary artery bypass graft surgery; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; NA, not available; PTCA, percutaneous transluminal coronary angioplasty; SBP, systolic blood pressure; TIA, transient ischemic attack.
In the HOPE study, 16% of the patients required an unblinded (open) angiotensin-converting enzyme inhibitor as well as the target treatment of the study. In the PRoFESS study, 37% of the patients were treated with an angiotensin-converting enzyme inhibitor (dual blockade) as well as the target treatment of the study.
Until the publication of the ONTARGET trial, there were no data comparing ARB and ACEI in high-risk cardiovascular patients beyond those with heart failure. When the populations of the ONTARGET, TRANSCEND, and PRoFESS trials are compared with those of the HOPE study, the patients in these 3 trials can be seen to have a higher prevalence of hypertension, stroke, and left ventricular hypertrophy (Table 3). In all these studies, the blood pressure values clearly indicate that the patients were relatively well controlled and, moreover, that most patients were receiving effective treatments, such as antiplatelets, lipid-lowering agents (statins), and beta-blockers (Table 3). In fact, a lower percentage of patients included in the HOPE trial received statins, probably reflecting the time at which the study was performed. Furthermore, when the results of the ONTARGET and HOPE trials are compared (Figure 3), it can be argued that high-risk patients with coronary artery disease that have been revascularized (>40% of the patients in the HOPE, ONTARGET, and TRANSCEND studies) and continue to be treated with antiplatelets and statins do not show a poor prognosis, which makes it difficult to obtain a clear additional clinical benefit after the introduction of a new treatment.12 Nonetheless, assessment of the occurrence of cardiovascular events in the control group (baseline risk) and in the intervention group in each group can illustrate in a general way the possible impact of the characteristics of the study and patients on the results of interest (Figure 3).
FINAL CONSIDERATIONSThe interpretation of clinical trial results (and their subsequent use in a meta-analysis or when making an informal indirect comparison) can be complicated if no consideration or critical assessment is made of aspects related to the study and patient characteristics that could act as effect-modifying factors. In the example presented, derived from large published clinical studies, if the results of a clinical trial of C (ARB) vs B (ACEI) are considered, the results of a clinical trial of A (placebo) vs B (ACEI) would not guarantee the correct inference in clinical trials of A (placebo) vs C (ARB) because transitivity does not hold. Understandably, heterogeneity13 continues to be seen as a problem to be controlled and explained. However, the reality of clinical practice is that it is performed in diverse populations with distinct demographic, clinical, and epidemiological characteristics. The complexities resulting from this situation should be accepted to ensure that clinical research has the highest clinical impact possible.14 For the same reason, with the information available at the time, efforts should be made to design rigorous studies that exhaustively evaluate the effects of diverse interventions or treatments that compete with one another for the same clinical indication, not only to determine which of them is in general superior or not inferior to the others, but also to investigate which patients can be candidates for a treatment or if a treatment might be better in different patient subgroups.
Indirect treatment comparisons based on individual clinical studies can contain considerable selection biases that seriously call into question the validity of the results obtained. Any indirect comparison between treatments should always be made in the context of systematic reviews and rigorous meta-analyses, taking into account a “complete network” of studies that guarantee their quality. Moreover, one of the advantages of systematic reviews is that they enable determination and quantification of possible sources of variability in study results. Thus, the clinician or researcher should first perform a qualitative analysis of the study and patient characteristics, with special emphasis on those characteristics potentially acting as effect-modifying factors. As previously mentioned, study and patient characteristics can influence results. In particular, the effect or response in the control group (baseline risk) can reflect the impact of some of these characteristics on the results of interest. For example, if a treatment only works in patients that tolerate or respond to a previous treatment (responders) and not in those that do not respond (refractory or resistant patients), a study conducted in responders will show a positive effect of the treatment relative to the control (placebo), whereas a study conducted only in those refractory or resistant will fail to show a positive effect. Another example is that if the result of interest (improvement in cardiovascular death, myocardial infarction, or stroke) is superior to a treatment in a trial performed a decade ago, it is possible that, due to differences in clinical practice (eg, greater intensity, concomitant use of other therapies) or study design, the period in which the study was performed can act as another effect-modifying factor.
Finally, evidence tables with the baseline characteristics and patient inclusion criteria of each study can provide information useful for critically assessing aspects that could affect the transitive property. Readers should rely on their experience and on their knowledge of the disease of interest, the treatments evaluated, and the designs of the relevant studies. Currently, sophisticated analysis methods are being developed and applied that enable recognition of some of these factors.1–3 Some of these techniques permit indirect comparisons to be made that adjust for the effects of diverse factors that can act as effect modifiers, thereby reducing potential sources of bias. The response of the control group (baseline risk) or the year of the study can be used as an adjustment variable in the analysis models, although this alternative approach is not without its limitations.15,16 However, these methodologies should also be supported by thorough evaluations and should be based on clinical-epidemiological grounds.
FUNDINGDrs. Hutton and Moher have received funding from CIHR/DSEN (Canadian Institutes of Health Research/Drug Safety and Effectiveness Network). Dr. Moher has received funding from the Research Chair of the University of Ottawa, Canada.
CONFLICTS OF INTERESTNone declared.
The opinions expressed in this editorial are those of the authors and do not necessarily reflect the views of the institutions in which they work.